Girish BN1, Rajesh G2, Vaidyanathan K3, Balakrishnan V2
Departments of Physiology1,
Gastroenterology2 and Biochemistry3
Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham
AIMS Ponekkara P.O. Cochin – 682 041
Kerala, India
Corresponding Author:
Prof. V. Balakrishnan Department of Gastroenterology,
Amrita Institute of Medical Sciences,
Amrita Vishwa Vidyapeetham AIMS Ponekkara P.O. Cochin – 682 041
Kerala, India
E-mail: vbalakrishnan@aims. amrita.edu
Abstract
Background: The role of trace elements in the maintenance of normal pancreatic function is unclear.
Aim: To estimate trace elements (zinc and copper) in chronic pancreatitis (CP) patients and study their relationship with exocrine and endocrine insufficiency. Methods: The study involved 101 CP patients and 113 healthy controls. Disease characteristics and imaging features were recorded. Erythrocyte zinc (Zn) and copper (Cu) were estimated by flame atomic absorption spectrophotometry. Fecal pancreatic elastase1 was estimated by polyclonal antibody ELISA method as a marker of pancreatic exocrine function.
Results: The mean erythrocyte Zn level and Zn/Cu ratio were significantly lower whereas the copper level was significantly higher in CP patients than controls. The mean Zn level and Zn/Cu ratio was significantly lower in CP patients with diabetes and those with low elastase1 as compared to non-diabetics and those with normal elastase1 respectively. Erythrocyte Cu level was significantly higher in CP patients with diabetes and with low elastase1 than those without diabetes and with normal elastase1 levels respectively. There was a significant positive correlation between elastase1 and Zn/Cu ratio (r = 0.396, p < 0.001). Receiver operating characteristic curve (ROC) analysis was performed to predict the development of exocrine insufficiency and it indicated an area under curve (AUC) of 0.838 ± 0.047 (95% CI: 0.746-0.93). The optimal cutoff value was 9.03 (sensitivity 86.5%, specificity 73.5%). When the same was performed to predict the development of diabetes, the AUC was 0.710 ± 0.05 (95% CI: 0.607-0.812). The optimal cutoff value was 7.2 (sensitivity 69.1%, specificity 69.7%).
Conclusion: Low erythrocyte Zn/Cu ratio was found to be associated with exocrine and endocrine insufficiency in CP patients.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1696 48uep6bbph|2000F98CTab_Articles|Fulltext Chronic pancreatitis (CP) is defined as a persistent inflammatory disease of the pancreas characterized by irreversible morphological changes typically causing pain and/or permanent loss of function which may be associated with exocrine and/or endocrine insufficiency[1]. The pathogenic mechanisms including the metabolic and genetic consequences appear to be multifactorial[2]. One such mechanism may be a disturbance in trace element levels. In our previous report we documented zinc deficiency in chronic pancreatitis patients as compared to healthy controls[3]. The pancreas is among the organs where the most rapid accumulation and turnover of retained zinc occurs. Therefore, it is likely that the pancreas is the most affected by zinc deficiency[4]. In our study, zinc deficiency correlated with pancreatic exocrine and endocrine insufficiency[3]. Recently, Yu et al reported that zinc deficiency was a common phenomenon in patients after pancreato-duodenectomy and it correlated with pancreatic exocrine insufficiency[5].
Copper is an essential trace element required as a cofactor for a variety of enzymes[6]. Although copper is required in trace amounts, it is toxic when present in excess[6]. Copper accumulates to toxic levels in cells in several human disorders, causing cellular injury and progressive tissue damage[7]. It has been widely recognized that trace amounts of transition metals including copper play a major role in oxidant-induced tissue injury through participation in the generation of hydroxyl radicals, which rapidly react with polyunsaturated fatty acid residues of cell membranes and facilitate lipid peroxidation[8].
Keeping in mind the relevance of these minerals in human metabolism especially pancreatic function, and taking into account the sparse reports in literature regarding zinc / copper (Zn/Cu) ratio in chronic pancreatitis, we propose to assess erythrocyte zinc and copper levels in CP patients and compare them with those in healthy controls. Zinc deficiency may affect oxidative status in CP patients[9].Hence we estimated the blood levels of enzymes that protect against oxidative stress. We also correlated the patients’ Zn/Cu ratio with the level of pancreatic exocrine insufficiency.
Methods
Subjects
Chronic pancreatitis patients were recruited from the Pancreas Clinic, Amrita Institute of Medical Sciences. Chronic pancreatitis was defined by features consistent with irreversible pancreatic inflammation, i.e., clinical, structural or functional abnormalities of the pancreas[10]. The presence of pancreatic calculi or ductal irregularity/ parenchymal atrophy was determined at imaging using ultrasonography, CT scan, MRI, magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP) or endoscopic ultrasound (EUS). Patients having chronic pancreatitis with an alcohol consumption equal to or greater than 80 g/day for at least 5 years were considered to have alcoholic chronic pancreatitis while tropical chronic pancreatitis was defined using previously reported criteria1. The study was approved by the institutional ethical review committee.
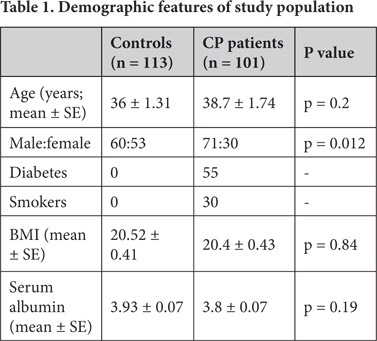
One hundred and one patients with chronic pancreatitis (34 alcoholic chronic pancreatitis, and 67 tropical chronic pancreatitis) were included and prospectively studied (Table 1).
Controls
One hundred and thirteen age matched controls (60 males, 53 females) were recruited from among healthy non-smoking hospital visitors. None of the patients or controls had frank diarrhea. Those subjects in whom a history and physical examination did not show any physical illness or symptoms were considered to be normal healthy controls.
Data Recording
Dietary details of each subject were collected and recorded. Subjects using vitamin and mineral supplements, or consuming fortified foods were excluded from the study. Disease characteristics such as pain, steatorrhea, diabetes mellitus and insulin requirement, risk factors such as alcohol and smoking as well as imaging (US/CT) features such as calculi, parenchymal atrophy and ductal dilation, were recorded. BMI was also calculated (weight in kg / height in m2).
Serum albumin was measured using bromocresol green[11]. Erythrocyte zinc and copper were estimated as they provide an assessment of zinc status over a longer period of time as compared to the plasma pool, where turnover was rapid[12]. Fasting blood samples were collected in heparinized vacutainers. RBCs were washed 3 times by resuspending the cells in cold normal saline and centrifuging (1,500 g at 4°C). The buffy coat was separated. The washed cells were lysed with two volumes of milli-Q water and frozen at -20°C. Hemoglobin concentration was measured using the cyanmethhemoglobin method. Erythrocyte lysate was diluted 10 fold with milli-Q water; zinc and copper concentrations were determined by flame atomic absorption spectrophotometry (3110, Perkin Elmer, Waltham, MA, USA)[13, 14] and values were expressed as micrograms of zinc per g of hemoglobin. Stool samples of chronic pancreatitis patients were collected and stored at -4°C for less than one week prior to use. Fecal pancreatic elastase1 was measured by using a polyclonal antibody-based ELISA kit (Bioserv, Rostock, Germany).
Fecal pancreatic elastase1 in moderate pancreatic exocrine insufficiency was 100 to 200 µg/g and, for severe exocrine insufficiency, it was less than, or equal to, 100 µg/g. Plasma fasting and postprandial glucose levels and insulin requirements were recorded to estimate endocrine insufficiency. Blood antioxidant, lipid peroxidation product – thiobarbituric acid-reactive substances (TBARS) and serum albumin were measured as reported earlier[15].
Diabetes mellitus was diagnosed if the fasting serum glucose value was equal to, or greater than, 126 mg/dL confirmed on two occasions and/or a serum glucose value equal to, or greater than, 200 mg/dL after a two-hour glucose load confirmed on two occasions, and/or the subject had requirement of insulin or oral hypoglycemic drugs.
Statistical analysis
Statistical analysis was conducted by using SPSS (version 11). Data was reported as mean±SE and frequencies. The Mann-Whitney U-test was performed to compare means. Linear correlation between the two groups was evaluated by calculating the Spearman rank correlation coefficient. The ROC curve was calculated to evaluate the accuracy of RBC Zn/Cu ratio in predicting low (200µg/g) fecal pancreatic elastase1; the area under the ROC curve was computed together with the standard error (AUC±SE) and the 95% CI. The optimal cut-off value which best predicted low elastase1 was calculated using a maximum likelihood ratio method[18]. Two-tailed P values less than 0.05 were considered statistically significant.
Results
The demographic characteristics of the study population are given in Table 1. Of the 101 chronic pancreatitis patients, 67 were tropical pancreatitis (TCP) patients (37 males and 30 females) and 34 were alcoholic chronic pancreatitis (ACP) patients (all males). Eighteen CP patients and three healthy control subjects were excluded from the study due to the use of vitamin / mineral supplements.
The mean age, BMI and serum albumin level were comparable between CP patients and controls (Table 1). Gender was not matched between CP patients and control subjects (Table 1). None of the normal controls were diabetics or smokers. Disease characteristics such as pain occurred in 83 % of CP patients, diabetes mellitus in 54.5 %, steatorrhoea in 66.3 % and 53 % of CP patients with diabetes required insulin. Imaging studies detected calculi in 66.2 %, ductal dilatation in 65 % and atrophy in 23.4 %.
Chronic pancreatitis patients showed significantly lower RBC zinc levels and Zn/Cu ratio than controls. While RBC copper levels were elevated in CP patients as compared to normal controls, blood antioxidants such as glutathione (GSH), glutathione peroxidase (GPX), superoxide dismutase (SOD) and vitamin C levels were significantly lower in CP patients. Lipid peroxidation product TBARS was significantly elevated in CP patients (Table 2).

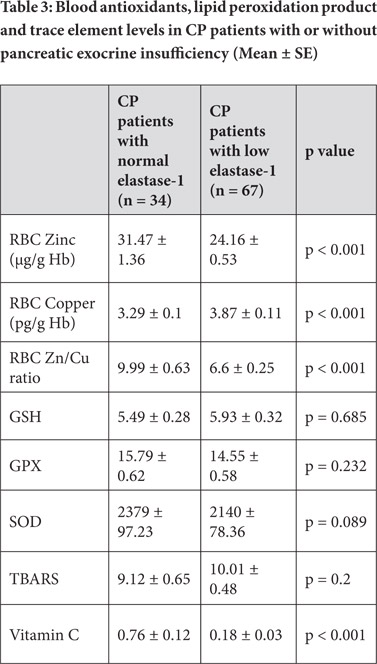
Erythrocyte zinc level and Zn/Cu ratio was significantly lower whereas erythrocyte copper was higher in CP patients with low elastase1 levels and diabetes as compared to patients with normal elastase1 and without diabetes respectively. There was no significant difference in antioxidant levels between CP patients with or without low elastase1 levels and diabetes.
However, plasma vitamin C level was significantly lower in CP patients with low elastase1 levels and diabetes as compared to that in those with normal elastase1 and without diabetes respectively (Tables 3 and 4).

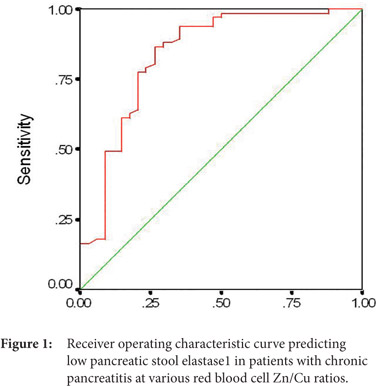
Fecal pancreatic elastase1 and erythrocyte Zn/Cu ratio were positively correlated (r = 0.396, p < 0.001). A receiver operating characteristic curve was plotted to evaluate the effectiveness of RBC Zn/Cu ratio in predicting pancreatic exocrine insufficiency (Figure 1). We obtained an area under the ROC curve (AUC±SE) of 0.838 ± 0.047 (95% CI: 0.746-0.93). At the best cut-off value of RBC Zn/Cu ratio (9.035), the sensitivity and specificity were 86.57% (58/67) and 73.53% (25/34), respectively.
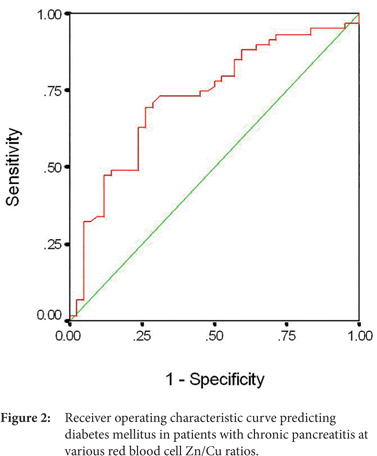
A receiver operating characteristic curve was plotted to evaluate the performance of RBC Zn/Cu ratio in predicting pancreatic endocrine insufficiency in the form of diabetes (Figure 2). We obtained an area under the ROC curve (AUC±SE) of 0.710 ± 0.05 (95% CI: 0.607-0.812). At the best cut-off value of RBC Zn/Cu ratio (7.2), the sensitivity and specificity were 69.1 % (38/55) and 69.56 % (32/46), respectively.
Discussion:
Chronic pancreatitis is a heterogeneous disease caused probably by a combination of genetic and environmental factors. Several factors are likely to influence the course of the disease. We have reported zinc deficiency in chronic pancreatitis patients[3]. Zinc deficiency was more pronounced in tropical pancreatitis and diabetic patients as compared to alcoholic chronic pancreatitis and non-diabetic patients[3].
The pancreatic acinar cells of zinc deficient rats showed reduction and rupture of zymogen granules[4]. Navarro et al showed that zinc deficiency is associated with collagen deposition and pancreatic fibrosis[16]. Supporting these findings, Geetha Armugam et al showed that reduced zinc levels and increased levels of copper and iron in the pancreatic tissue resected from CP patients may contribute to collagen deposition during the process of calcification[17]. Recently, ZnT2 (SLC30A2) has been implicated in Zn transport into zymogen granules of the pancreas which is required for the functioning of various proenzymes. Zn deficiency decreases ZnT2 expression and Zn concentration in zymogen granules and thereby can decrease pancreatic enzyme activity[18]. Hsin-Hsien Yu et al reported zinc deficiency in patients after pancreatoduodenectomy[19]. It was pointed out that zinc deficiency in these patients may be due to hypoproteinemia that produces lack of carrier protein for zinc transportation. Symptoms of zinc deficiency and gastrointestinal disorder improved after pancreatic enzyme supplementation. Qin-qing Tang et al showed that zinc supplementation in rats with severe acute pancreatitis decreased free radical content in the pancreas as well as in extrapancreatic tissues such as the liver and kidney which also displayed much evidence of zinc supplementation[20].
Zinc deficiency is more frequently observed in pancreases with adenocarcinoma as compared to normal/ benign pancreatic tissue. A decrease in zinc, RREB1 transcription factor and ZIP3 zinc uptake transporter levels appears to be an early event in the development of pancreatic cancer. Franklin et al demonstrated that exposure of Panc1 cells to physiological concentrations of zinc results in increased zinc uptake and its accumulation also inhibits cell proliferation[21].
Absorption of zinc from the intestine may be hampered in patients of pancreatitis. Ijuin et al studied zinc absorption in alcoholism using a combination of zinc tolerance tests on 382 male patients with alcoholic liver cirrhosis and chronic pancreatitis22. Zinc absorption was significantly decreased in these patients. The administration of zinc dipicolinate elevated serum zinc levels suggesting that chronic reduction of ligands such as picolinic acid synthesized in the liver are involved in decreased zinc absorption in chronic alcoholism[22].
In our study we observed a significant increase in erythrocyte copper levels in CP patients. Our finding was in agreement with Bell et al, Fabris et al and Segal et al[23,24,25]. It is well established that copper can serve as a pro-oxidant by catalyzing the production of hydroxyl
radical via Fenton reaction. It was suggested that copper ions bind to sulfhydryl groups in cells and inactivate the action of glucose-6-phosphate dehydrogenase and glutathione reductase, both of which are necessary for the reduction of oxidized glutathione to the reduced form of glutathione[26]. These effects would tend to decrease the reduced glutathione content of erythrocytes and make them more vulnerable to the oxidative effects of copper. It has been also demonstrated that copper accumulation may result in cellular damage and apoptosis[27].
Braganza et al showed that there is increased absorption of copper in patients with chronic pancreatitis[28]. About 90% of the copper in the blood is incorporated into ceruloplasmin, which is responsible for carrying copper to tissues. Inflammatory reactions increase ceruloplasmin levels. The increased copper concentrations probably reflected an increase in ceruloplasmin concentrations as part of an inflammatory syndrome[29].
The levels of antioxidants such as glutathione, glutathione peroxidase, superoxide dismutase and vitamin C were significantly lower in CP patients. However, on subgroup analysis of patients with or without low elastase1 and diabetes, there was no significant difference except for vitamin C levels. Segal et al observed an inverse relationship between ceruloplasmin levels and vitamin C levels in CP patients[25].
The blood concentrations of zinc appear to be slightly affected by the levels of other nutrients such as copper. Copper can compete with zinc in the small intestine and interfere with its absorption[30]. During inflammatory conditions there are other mechanisms that act to decrease blood zinc levels and increase copper levels[31]. Hence in addition to the measurement of zinc and copper alone, particular attention should be drawn to Zn/Cu ratio. This study shows that there is a significant association between Zn/Cu ratio and fecal pancreatic elastase1 levels which are a marker of pancreatic exocrine function. A limitation of this study is that it has a cross-sectional design, implicating that cause and effect relationships cannot be discerned. Streptozotocin- induced diabetic rats showed a significant decrease in tissue zinc to copper ratio which seems to be dependent on the duration of disease[32]. Milnerowicz et al observed lowered serum Zn concentration and higher Cu level in patients with chronic exacerbated pancreatitis and it was indicated that the disturbance in zinc and copper homeostasis depends on the progression of the inflammatory process in patients with pancreatitis[33]. Karahan SC et al suggested that Cu/Zn ratio may be used as an important marker to evaluate the presence of vascular complications in diabetic patients[34]. We previously reported hyperhomocysteinemia in CP patients[35]. Hyperhomocysteinemia is known to hamper vascular integrity and predispose to vascular thrombosis.
In conclusion, we showed reduced zinc levels and increased copper levels in CP patients. There is a significant association of Zn/Cu ratio with pancreatic exocrine and endocrine insufficiency supporting the idea that Zn/Cu ratio may be used as a biomarker for exocrine pancreatic dysfunction in pancreatitis patients. Further study is warranted to test Zn/Cu ratio in different age groups since old age can disturb the metabolism of trace elements.
References
- Balakrishnan V, Unnikrishnan AG, Thomas V, et al. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP. 2008;9:593–600.
- Balakrishnan V. Tropical chronic pancreatitis: a historical perspective. Gut. 2011;60:1441.
- Girish BN, Rajesh G, Vaidyanathan K, et al. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP. 2009;10:651-6.
- Koo SI, Turk DE. Effect of zinc deficiency on the ultrastructure of the pancreatic acinar cell and intestinal epithelium in the rat. J Nutr. 1977;107:896-908.
- Yu HH, Yang TM, Shan YS, et al. Zinc deficiency in patients undergoing pancreatoduodenectomy for periampullary tumors is associated with pancreatic exocrine insufficiency. World J Surg. 2011;35:2110-7.
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S-811S.
- Scheinberg IH, Sternlieb I.Wilson’s Disease. In: Lloyd H, Smith J, editors. Major Problems in Internal medicine. WB Saunders: Philadelphia; 1984. pp 25–37
- Halliwell B, Gutteridge JM.Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1-14.
- Girish BN, Rajesh G, Vaidyanathan K,et al. Assessment of oxidative status in chronic pancreatitis and its relation with zinc status. Indian J Gastroenterol. 2011;30:84-8.
- Etemad B, Whitcomb DC. Chronic pancreatitis:Diagnosis, classification and new genetic developments. Gastroenterology. 2001;120:682-707.
- Burtis CA, Ashwood ER, Tietz NW, editors. Philadelphia, PA: W.B. Saunders; 1986. Tietz text book of clinical chemistry; p. 589.
- Gibson RS, Hess SY, Hotz C,et al. Indicators of zinc status at the population level:a review of the evidence. Br J Nutr 2008; 99:S14-S23.
- Kenney MA, Ritchey SJ, Culley P,et al. Erythrocyte and dietary zinc in adolescent females. Am J Clin Nutr 1984; 39:446-51.
- Blomfield J, Macmahon RA. Micro determination of plasma and erythrocyte copper by atomic absorption spectrophotometry. J Clin Pathol. 1969;22:136-43.
- Girish BN, Rajesh G, Vaidyanathan K, et al. Fecal elastase1 and acid steatocrit estimation in chronic pancreatitis. Indian J Gastroenterol. 2009 ;28:201-5.
- Navarro S, Valderrama R, To-Figueras J, et al. Role of zinc in the process of pancreatic fibrosis in chronic alcoholic pancreatitis. Pancreas. 1994;9:270-4.
- Arumugam G, Padmanaban M, Krishnan D, et al. Influence of copper, iron, zinc and fe (3) (+) haemoglobin levels on the etiopathogenesis of chronic calcific pancreatitis--a study in patients with pancreatitis. Biol Trace Elem Res. 2011;142:424-34.
- Kelleher SL, McCormick NH, Velasquez V, et al. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011;2:101-11.
- Yu HH, Yang TM, Shan YS, et al. Zinc deficiency in patients undergoing pancreatoduodenectomy for periampullary tumors is associated with pancreatic exocrine insufficiency. World J Surg. 2011;35:2110-7.
- Tang QQ, Su SY, Fang MY. Zinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitis. Biol Trace Elem Res. 2014;159:320-4.
- Franklin RB, Zou J, Costello LC. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol Ther. 2014;15:1431-7.
- Ijuin H. Evaluation of pancreatic exocrine function and zinc absorption in alcoholism. Kurume Med J. 1998;45:1-5.
- Bell M, Jamison M, Braganza JM. Serum copper oxidase activity (coeruloplasmin) in chronic pancreatitis: inverse correlation with pancreatic exocrine function. Clin Chim Acta. 1981;117:259-68.
- Fabris C, Farini R, Del Favero G, et al. Copper, zinc and copper/zinc ratio in chronic pancreatitis and pancreatic cancer. Clin Biochem. 1985;18:373-5.
- Segal I, Sharer NM, Kay PM, et al. Iron, ascorbate and copper status of Sowetan Blacks with calcific chronic pancreatitis. QJM. 1996 ;89:45-53.
- Attri S, Sharma N, Jahagirdar S, et al. Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res. 2006;59:593-7.
- Saravu K, Jose J, Bhat MN, et al. Acute ingestion of copper sulphate: A review on its clinical manifestations and management. IJCCM. 2007; 11:74-80.
- Braganza JM, Klass HJ, Bell M, et al. Evidence of altered copper metabolism in patients with chronic pancreatitis. Clin Sci (Lond). 1981;60:303-10.
- Morris-Stiff GJ, Bowrey DJ, Oleesky D, et al. The antioxidant profiles of patients with recurrent acute and chronic pancreatitis. Am J Gastroenterol. 1999;94:2135-40.
- Oestreicher P, Cousins RJ. Copper and zinc absorption in the rat: mechanism of mutual antagonism. J Nutr. 1985 ;115:159-66.
- Zoli A, Altomonte L, Caricchio R, et al. Serum zinc and copper in active rheumatoid arthritis: correlation with interleukin 1 beta and tumour necrosis factor alpha. Clin Rheumatol. 1998;17:378-82.
- Aguilar MV, Laborda JM, Martínez-Para MC, et al. Effect of diabetes on the tissular Zn/Cu ratio. J Trace Elem Med Biol. 1998;12:155-8.
- Milnerowicz H, Jablonowska M, Bizon A. Change of zinc, copper, and metallothionein concentrations and the copper-zinc superoxide dismutase activity in patients with pancreatitis. Pancreas. 2009;38:681-8.
- Karahan SC, Deger O, Orem A, et al. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med. 2001;39:109-15.Girish BN, Vaidyanathan K, Rao NA, et al. Chronic pancreatitis is associated with hyperhomocysteinemia and derangements in transsulfuration and transmethylation pathways. Pancreas. 2010;39:e11-6.
|