Vishal Sharma, Ujjawal Gorsi*, Rajesh Gupta**, Surinder S Rana***
Department of Gastroenterology,
Radiodiagnosis*& Surgery**,
Post Graduate Institute of Medical Education and Research (PGIMER), Sector 12,
Chandigarh – 160012, India
Corresponding Author:
Dr Surinder Singh Rana
Professor, Department of Gastroenterology
PGIMER, Chandigarh – 160 012, India
E-mail: drsurinderrana@gmail.com
Abstract
Acute
pancreatitis (AP) is a disease of varying severity with acute
necrotizing pancreatitis (ANP) being the severe form of AP. ANP needs
multidisciplinary management and is associated with significant
mortality and morbidity. Local complications of ANP viz. acute necrotic
collections (ANC) and walled off pancreatic necrosis (WOPN) need
intervention if they are associated with symptoms, sepsis or worsening
organ failure. As surgery is associated with higher morbidity and
mortality, there is paradigm shift in the management of these local
complications with an increased emphasis on conservative and minimally
invasive treatment approach. Image-guided percutaneous drainage (PCD) is
a minimally invasive intervention that helps in drainage of infected
collections, temporizes sepsis and improves outcomes in these patients.
The present review discusses the utility of various percutaneous
interventions in management of variety of pancreatic collections
occurring in ANP. The available evidence, technical details and
complications and emerging innovations in the field are also summarized.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1695 48uep6bbph|2000F98CTab_Articles|Fulltext Acute Pancreatitis (AP) is an acute inflammatory disease of the pancreas, which is characterized by the frequent development of local or systemic complications. It is broadly divided into two morphological patterns: interstitial, which is merely associated with edema of the pancreatic parenchyma or necrotizing, in which peri-pancreatic and/or pancreatic necrosis is present.[1-3] In the early phase of acute pancreatitis the heightened inflammatory response dominates the clinical picture while the late phase is characterized by development of local infective as well as non-infective complications. The pancreatic inflammation results in exudation of pancreatic juice and subsequent injury to the peri-pancreatic fat and tissues.[2,3] This results in formation of collections which may be associated with both interstitial and necrotizing pancreatitis. While the collections formed in relation to interstitial pancreatitis are predominantly fluid and are called as acute fluid collections (AFC), those in association with necrotizing pancreatitis are labelled as acute necrotic collections (ANC) since they also contain solid necrotic debris apart from the pancreatic juice and fluid. Over time these fluid collections develop a non-epithelial fibrinous wall around them, gradually liquefy and are then labelled as pseudocysts or walled off pancreatic necrosis (WOPN) respectively.[4,5] AFC associated with interstitial pancreatitis resolve or form pseudocysts which are fluid containing collections without any necrotic material. In contrast, the walled off pancreatic necrosis (WOPN) is usually a consequence of necrotizing pancreatitis and results from formation of a wall around acute necrotic collections[1]
Indications for drainage of pancreatic fluid collections (PFC)
The PFC’s are usually managed with a “wait and watch” strategy. The dictum is not to intervene unless symptoms from pancreatic fluid collections necessitate drainage. Depending on the location of pancreatic collections the symptoms may include abdominal pain, obstruction of the gastric outlet or biliary obstruction. The collections may get infected and cause fever and features of sepsis. In early phase of acute severe pancreatitis, worsening organ failure and development of abdominal compartment syndrome may warrant drainage of PFCs even when no abdominal pain or clear evidence of infection is present.[6] The choice of the modality of drainage is dependent upon a number of factors including the presence of a wall around the collection, distance of the collection from the gastrointestinal lumen and presence and extent of any solid debris.[7] As long as the collections in early pancreatitis do not have a wall, they are best drained percutaneously using radiology guided interventions. Surgery is not preferred in early phase as the collections have not yet organized and surgery is associated with higher morbidity and mortality. However, in the late phase, usually after 4 weeks, the collections develop a wall around them and then endoscopic management is feasible. The endoscopic drainage using direct endoscopic drainage or endoscopic ultrasound (EUS) guided drainage is usually preferred as it is less invasive and obviates the risk of external pancreatic fistula (EPF) that is associated with percutaneous drainage (PCD).8 The availability of self-expanding metallic stents (SEMS) for endoscopic drainage of PFCs have improved the outcome of endoscopic drainage of walled off necrotic collections. However, endoscopic drainage may not be feasible in a subset of patients when the collections are distant from the gastrointestinal lumen or if the collections have not organized with a well formed wall. In these situations, PCD or surgery may be resorted to help manage the patient. This review will focus only on percutaneous interventions for ANP and readers are advised to consult other reviews on endoscopic or surgical management of ANP.[7,8]
Technique of Percutaneous Drainage (PCD)
Pre procedure evaluation: Informed consent needs to be obtained from the patient and imaging studies and laboratory parameters should be reviewed prior to procedure to plan the route of drainage. Patient’s coagulation parameters should be within acceptable limits prior to procedure with platelet count of at least 50,000/µL and international normalized ratio (INR) less than 1.5. Equipment for monitoring and resuscitation must be available at the time of procedure. PCD is usually done under local anesthesia with conscious sedation but rarely general anesthesia may be required, especially while putting wide bore catheters.
Image Guidance: In collections related to pancreatitis, computed tomography (CT) is the preferred modality to guide drainage as frequently these collections are associated with paralytic ileus and are retroperitoneal in location. Ultrasound guidance may be used in large superficial collections near abdominal wall. Fluoroscopy may be used for serial dilatation and catheter placement following successful needle access.
Access routes: For collections in or near the pancreatic head and body usual route is through gastro-colic ligament (Fig.1a, 1b) whereas for collections in or near the pancreatic tail the most commonly used route is through the left anterior para-renal space. (Fig.2)
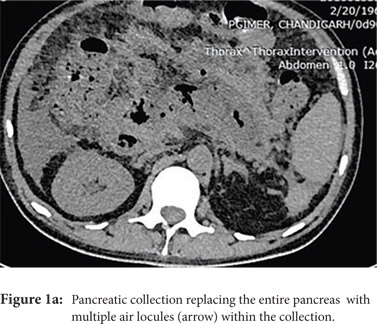
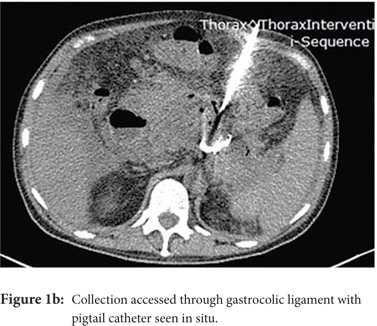
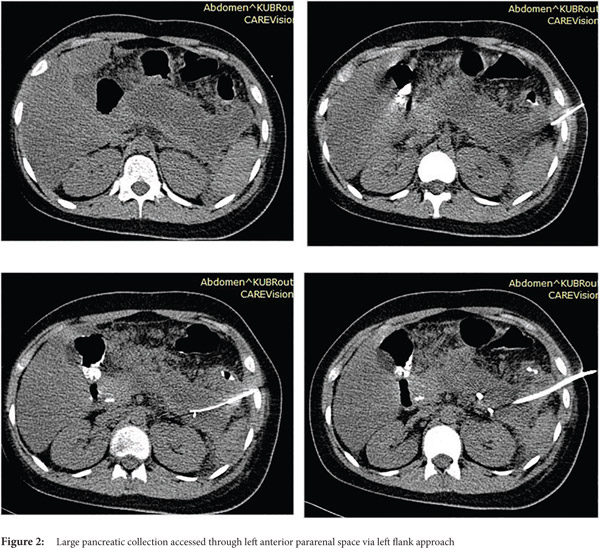 Fine needle aspiration(FNA): FNA is used to obtain a sample for microbiologic analysis in suspected infection. Fine (20-21G) needle is advanced into the most liquefied portion of the collection for collection of the sample. In the current era of symptom guided drainage of PFC’s as well risk of secondary infection of the collection following FNA, image guided FNA for documenting of infection is rarely needed.
Percutaneous drainage (PCD): Either the Seldinger or the trochar technique is used, depending on the size, location and expertise of the operator.
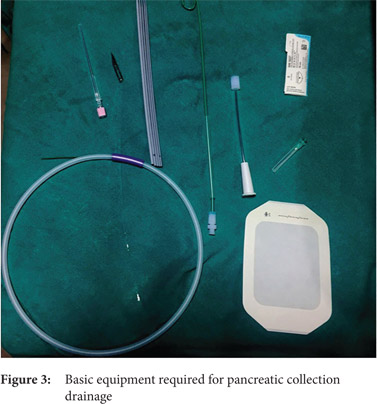
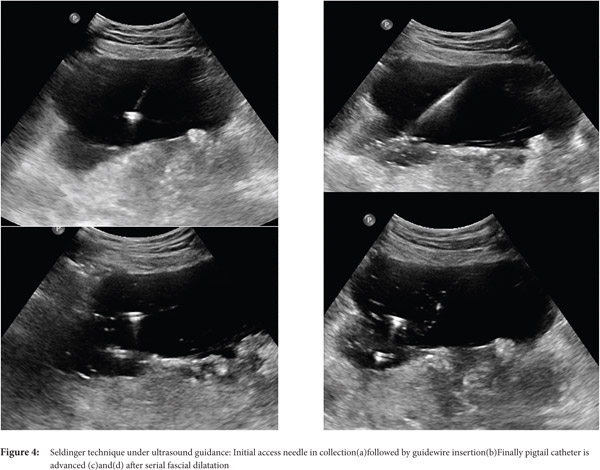
Seldinger technique: With the Seldinger technique, collection is initially accessed by 18G needle followed by insertion of 0.035 inch hydrophilic stiff guide wire. Then, serial dilatation is performed using fascial dilators followed by insertion of a pigtail catheter (Fig. 3 and 4)
Fine needle aspiration(FNA): FNA is used to obtain a sample for microbiologic analysis in suspected infection. Fine (20-21G) needle is advanced into the most liquefied portion of the collection for collection of the sample. In the current era of symptom guided drainage of PFC’s as well risk of secondary infection of the collection following FNA, image guided FNA for documenting of infection is rarely needed.
Percutaneous drainage (PCD): Either the Seldinger or the trochar technique is used, depending on the size, location and expertise of the operator.
Seldinger technique: With the Seldinger technique, collection is initially accessed by 18G needle followed by insertion of 0.035 inch hydrophilic stiff guide wire. Then, serial dilatation is performed using fascial dilators followed by insertion of a pigtail catheter (Fig. 3 and 4)
 Trocar technique: With the trocar technique, the collection is initially accessed using a smaller gauge needle followed by advancing of coaxial combination of a catheter, stiffening cannula, and sharp stylet into the collection.
Seldinger technique is useful for small deep, high risk, and difficult-to-access collections but it is more time consuming whereas the trocar technique is often used for large and superficial collections. Trocar technique is quicker but is usually more painful.
Catheter selection: For more liquid and less viscus collections, 10F -12 F catheters usually suffice where as large bore catheters ranging from 14F to 28F are required for collections having solid necrotic debris.
General principles for PCD of pancreatic collection:
Trocar technique: With the trocar technique, the collection is initially accessed using a smaller gauge needle followed by advancing of coaxial combination of a catheter, stiffening cannula, and sharp stylet into the collection.
Seldinger technique is useful for small deep, high risk, and difficult-to-access collections but it is more time consuming whereas the trocar technique is often used for large and superficial collections. Trocar technique is quicker but is usually more painful.
Catheter selection: For more liquid and less viscus collections, 10F -12 F catheters usually suffice where as large bore catheters ranging from 14F to 28F are required for collections having solid necrotic debris.
General principles for PCD of pancreatic collection:
- Use safest, shortest and most direct path for drainage.
- Place catheter in the most dependent portion of collection.
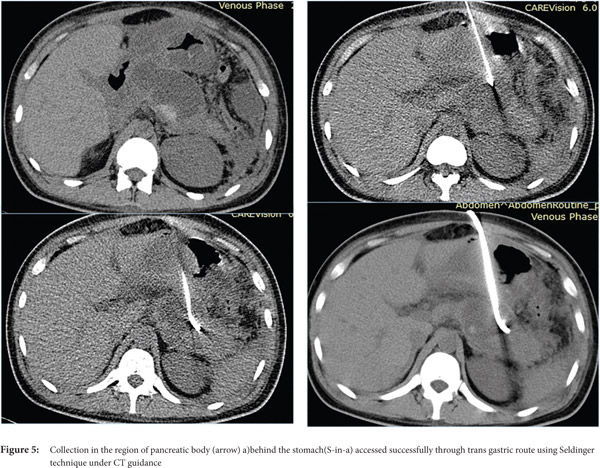
- Avoid transgressing vital structures. Organs that can be safely traversed are stomach (Figure 5) and liver, whereas organs such as pancreas, gall bladder, small/ large bowel and spleen should not be traversed.
- Frequent catheter upgradations are usually required in collections with solid debris.
- Multiple catheters may be required to facilitate drainage especially if collection is multi-loculated and locules are non-communicating.
- Frequent follow up imaging, replacement and repositioning are required as catheter drainage may continue for weeks.
Catheter removal- Catheter should be removed when there is resolution of clinical and laboratory findings and there is either no drainage, or drainage is less than 10 ml per day for at least three consecutive days. Also, there should not be any collection on imaging.
Complications of PCD:
- Dislodging or clogging of catheter
- Contamination of sterile necrosis during aspiration
- Pseudoaneurysm resulting from vascular injury
- Fistulous communication to the intestine
- External pancreatic fistula (EPF)
Complications can be minimized by:
- Correcting any existing bleeding tendency-coauglopathy or low platelet count
- Careful selection of access routes and by avoiding traversing vital organs
- Good catheter care and using locking catheters to prevent dislodgement of catheters.
Percutaneous drainage (PCD) for organized collections
(Pseudocysts and WOPN)
While pseudocysts and WOPN are commonly drained endoscopically, some reports have described the use of PCD in these situations. Most of the earlier reports describe percutaneous drainage primarily in pseudocysts as the nomenclature and distinction between pseudocysts and WOPN was not as well recognized at that time. In these reports, PCD was resorted to in patients with infected collection for urgent drainage and was associated with prolonged hospital stay, lesser success rate, and higher complications.[9,10] Reports differ regarding effects on mortality with conflicting results.[10,11] These differences in outcome amongst various studies possibly relate to patient selection for a given treatment regimen. The success of PCD therapy is possibly related to underlying ductal anatomy. In a report on 50 patients who underwent PCD for pancreatic pseudocysts, those with normal pancreatic ducts and those with strictures but no cysto-ductal communication had a shorter duration of PCD drainage.[12] Ductal anatomy and presence of chronic pancreatitis predicted the length and success of percutaneous drainage. Overall in pancreatic pseudocysts and walled off collections percutaneous drainage may be useful only in emergent situations when endoscopic or surgical drainage is not feasible.[13]
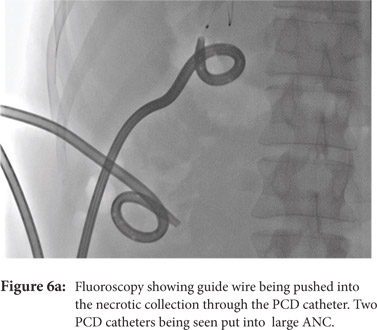
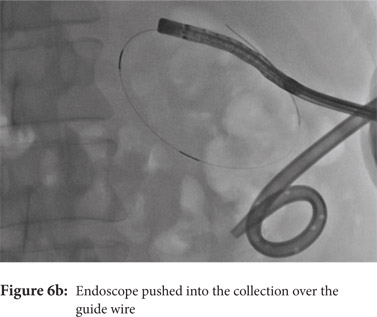
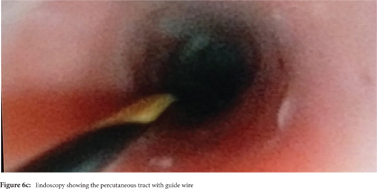
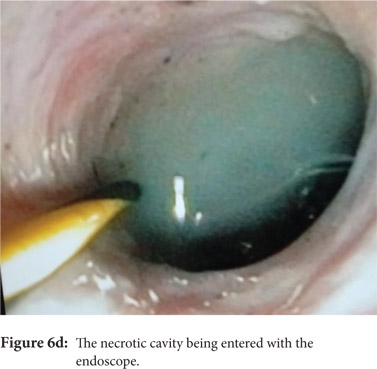
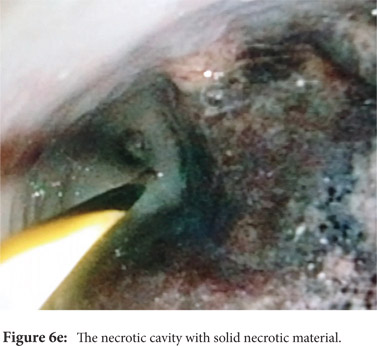
Some reports have also described utility of PCD in management of walled off necrosis. Most reports describe the use of percutaneous endoscopic necrosectomy (PEN) or sinus tract endoscopy through the tract of the percutaneous drain in order to remove the solid/necrotic debris (Figure 6 a-e). In a report of 12 patients treated with PEN, 10 patients had underlying WOPN. This involved upsizing of the percutaneous drains to 24-28 Fr, followed by insertion of endoscope under moderate sedation. Saline lavage was then done via the scope and debris removed using lavage or polypectomy snares. The mean number of sessions needed were 2.3 (range: 1-5) and fistula developed in one patient.[14] In another retrospective report from India, use of single or multiport percutaneous necrosectomy resulted in improvement in 14 of the 15 patients after a mean 5 sessions while one patient needed surgery and eventually died.[15] In another report from China, 13 patients with extensive pancreatic necrosis were managed by initial PCD or necrosectomy followed by sinus tract endoscopy. The mean number of sessions needed were 3 (range: 2-8), and the management failed in 3 of the 13 patients. Of these three patients, two were operated while the third died of sepsis.[16]
In a matched cohort study of 12 patients who underwent PCD as part of a step up approach for WOPN, comparison was made to a similar number of patients who underwent direct endoscopic necrosectomy (DEN).[17] Clinical success was reported in a higher number of patients in DEN group (11 patients versus 3 patients). Nine (75%) of PCD patients needed surgery and 7 of these witnessed a complicated course. This study, however, is a non-randomized study and may not have included patients of similar severity in each arm. Overall, because of substantial risk of fistula formation PCD alone is less preferable to endoscopic management in patients with WOPN but may be resorted to tide over emergency situation like infected WOPN in patients who have sepsis and are hemodynamically unstable.
PCD for Sterile Pancreatic Collections (Table 1)
The role of PCD in sterile acute collections is uncertain although some reports have examined the utility in these situations. The argument in favour of draining sterile collections comes from a Wistar rat model study where 60 rats were randomized to no intervention, open surgery and PCD group and it was noted that the levels of inflammatory cytokines were higher in the no intervention group in comparison to the PCD group.[18] Histological examination also suggested the role of PCD in alleviation of pancreatic injury.
A study reported about the early use of PCD in sterile collections related to severe acute pancreatitis in a group of 10 patients. While the study had only a small number of patients, it demonstrated that surgery was avoided in 4 patients. A reduction in levels of serum C reactive protein levels and total leucocyte count was demonstrated which suggested that the technique could help control the heightened systemic inflammatory response syndrome in these patients. However, no change was demonstrated in levels of TNF-alpha. In absence of a comparative arm, it is not possible to surely assert that the four patients who avoided surgery would have needed surgery in absence of PCD.[19] Another study reported a retrospective comparison of percutaneous aspiration versus drainage for sterile PFCs. While 15 patients underwent aspiration of small amount of fluid (10-20 ml), 22 patients underwent percutaneous drainage. No difference was noted in hospital stay or mortality in either of the group but those who underwent aspiration were more likely to undergo surgery or percutaneous drainage. The study also underscored the concern of catheter-induced infection and around half of the patients who underwent drainage developed infection.[20]
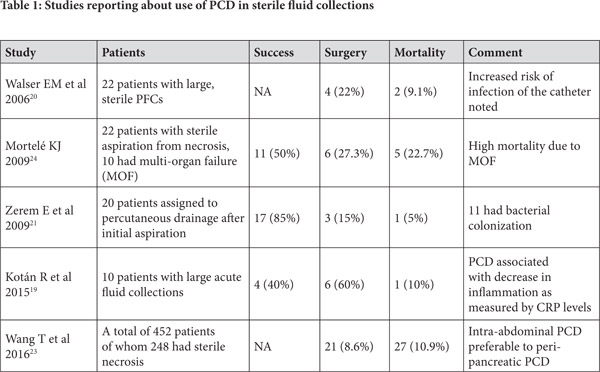 In a randomized trial comparing 40 patients of sterile fluid collections half of whom underwent fluid aspiration followed by conservative management while other half underwent percutaneous drainage, conservative management succeeded only in patients with single collection which was less than 30 ml while larger (>100 ml) or multi-loculated collections needed aggressive management. However, no direct comparison of conservative approach without initial aspiration versus drainage is available.[21] A consensus opinion published in 2012 suggested that sterile pancreatic collections do not need to be drained early in course.[22] However, the question whether these collections, even if sterile, be drained in patients with worsening organ failure and abdominal compartment syndrome remains unsettled and further controlled studies are needed to answer this question.
A study from China suggested that the drainage of intra-abdominal sterile pancreatic fluid collections is likely to be beneficial by reduction of abdominal pressure. Reduction of intra-abdominal pressure after PCD correlated with reduction in incidence of infection and improvement in organ failure.[23] In a retrospective comparison comparing 212 patients with acute pancreatitis who underwent PCD and 61 patients who underwent laparotomy with temporary closure for abdominal compartment syndrome, the need for post-procedure intensive care unit (ICU) support, and complication rates were lower in the PCD group. The mortality rates were lower in the PCD group (18.9%) versus the open laparotomy group (52.5%). This may therefore suggest the use of PCD drainage of collections as the initial approach in AP related abdominal compartment syndrome.6 In one report, however, the patients with sterile necrosis who underwent PCD fared worse than those with infected necrosis. This was probably on account of higher incidence of organ failure in these patients.[24]
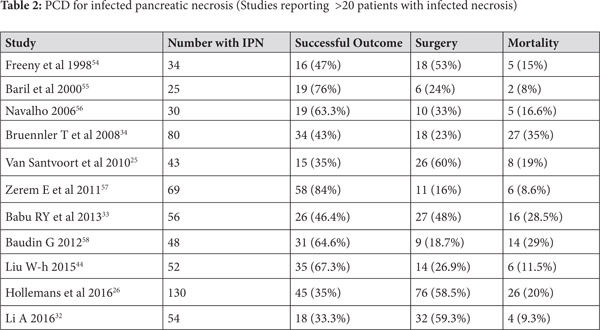
Use of PCD in infected ANC (Table 2)
Step-up approach
Use of percutaneous drainage of PFCs as a component of step-up approach for management of necrotizing pancreatitis was established by the PANTER Trial[25]. This randomized trial enrolled 88 patients with infected pancreatic necrosis and compared the traditional open necrosectomy with a step up approach, which utilized initial percutaneous drainage followed by minimally invasive necrosectomy. Herein the PCD was used to drain infected pancreatic collections initially and tide over the sepsis. The trial indicated that the minimally invasive approach which utilized PCD was superior, as the primary end point (a combination of complications like bleeding, fistula, perforation, organ failure and mortality) occurred in only 40% patients in the step up arm while it was recorded in 69% patients in the open necrosectomy group. Moreover, almost a third of patients in the step-up approach could successfully avoid surgical necrosectomy. The occurrence of incisional hernia, diabetes mellitus and need for pancreatic enzymes at 6 months was higher in the open necrosectomy group suggesting benefit with use of minimally invasive approach.[25] In another study of 130 patients with infected pancreatic necrosis treated with catheter drainage (predominantly percutaneous), success was achieved in 45 (35%) patients. The factors which suggested failure of PCD in this study were male gender, presence of multiple organ failure, amount of pancreatic necrosis and the morphology of collection on imaging.[26] Another report studied 35 patients with AP who underwent PCD of whom only 13 had infected pancreatic necrosis. Six patients of those with infected necrosis improved with PCD alone, 7 needed surgery while one died. In contrast, of the 22 patients with sterile necrosis 11 improved with PCD alone, 6 needed surgery and 5 died. The difference is possibly related to higher number of patients having multi-organ failure in the sterile necrosis group.[24] In another large study of 60 patients who underwent PCD, the major clinical indication was sepsis in 38 (63%) patients followed by pain in 20 (33%) and biliary obstruction in 2 patients. Overall success was achieved in 54 (90%) patients and three patients needed surgery whereas three patients died.[27]
Multiple systematic reviews have addressed the issue of use of PCD in the management of infected pancreatic necrosis. A recent systematic review included 15 articles reporting data on 577 patients of whom 352 patients had evidence of organ failure before insertion of PCD. Clinical success of PCD was defined as improvement without any further surgical intervention and was achieved in 56.2% (324) patients.[28] While 38.5% of patients needed additional intervention after initial PCD, overall mortality was 18% (104 patients). The major complications recorded were fistulae, organ failure and bleeding. The catheter size used in various studies was variable (6-32 Fr), and some studies reported upsizing of catheters and need for catheter changes. A Cochrane review of interventions in ANP suggested that use of step-up approach resulted in reduction of adverse events and lower costs vis-à-vis open necrosectomy.[29] Many other studies have also reported about the efficacy of PCD as a part of step-up approach in management of infected pancreatic necrosis
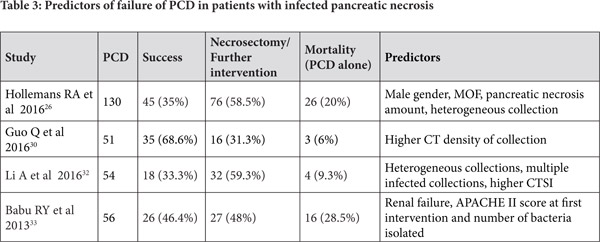
Predictors of clinical success for PCD in ANC
Some studies have attempted to identify predictors of success of PCD for infected pancreatic necrosis. In a report of 286 patients with AP who needed intervention, 51 were treated initially with PCD whereas remaining 235 patients underwent surgery. Of the 51 patients initially treated with PCD, 16 (31%) further needed surgery as part of step up approach and the only predictor of failure of PCD was computed tomographic (CT) density of the fluid collection.[30] In a retrospective report of 80 patients with severe AP who underwent placement of PCD, 58 patients had infected ANC. While the CT severity index (CTSI) and modified CTSI (MCTSI) scores did not predict mortality, the CT findings of a higher number of areas of necrosis in the pancreas, presence of distant fluid collections, and posterior para-renal space collections were associated with an increased risk of mortality.[31] The other factors that have been reported by various authors that may predict failure of PCD are enumerated in Table 3. These include organ failure, multiple infected collections and multi-organ infections, amongst others.[26,30,32,33] The importance in recognizing patients who are likely to fail PCD is to timely institute more invasive treatment options in these patients.
In a randomized trial comparing 40 patients of sterile fluid collections half of whom underwent fluid aspiration followed by conservative management while other half underwent percutaneous drainage, conservative management succeeded only in patients with single collection which was less than 30 ml while larger (>100 ml) or multi-loculated collections needed aggressive management. However, no direct comparison of conservative approach without initial aspiration versus drainage is available.[21] A consensus opinion published in 2012 suggested that sterile pancreatic collections do not need to be drained early in course.[22] However, the question whether these collections, even if sterile, be drained in patients with worsening organ failure and abdominal compartment syndrome remains unsettled and further controlled studies are needed to answer this question.
A study from China suggested that the drainage of intra-abdominal sterile pancreatic fluid collections is likely to be beneficial by reduction of abdominal pressure. Reduction of intra-abdominal pressure after PCD correlated with reduction in incidence of infection and improvement in organ failure.[23] In a retrospective comparison comparing 212 patients with acute pancreatitis who underwent PCD and 61 patients who underwent laparotomy with temporary closure for abdominal compartment syndrome, the need for post-procedure intensive care unit (ICU) support, and complication rates were lower in the PCD group. The mortality rates were lower in the PCD group (18.9%) versus the open laparotomy group (52.5%). This may therefore suggest the use of PCD drainage of collections as the initial approach in AP related abdominal compartment syndrome.6 In one report, however, the patients with sterile necrosis who underwent PCD fared worse than those with infected necrosis. This was probably on account of higher incidence of organ failure in these patients.[24]
Use of PCD in infected ANC (Table 2)
Step-up approach
Use of percutaneous drainage of PFCs as a component of step-up approach for management of necrotizing pancreatitis was established by the PANTER Trial[25]. This randomized trial enrolled 88 patients with infected pancreatic necrosis and compared the traditional open necrosectomy with a step up approach, which utilized initial percutaneous drainage followed by minimally invasive necrosectomy. Herein the PCD was used to drain infected pancreatic collections initially and tide over the sepsis. The trial indicated that the minimally invasive approach which utilized PCD was superior, as the primary end point (a combination of complications like bleeding, fistula, perforation, organ failure and mortality) occurred in only 40% patients in the step up arm while it was recorded in 69% patients in the open necrosectomy group. Moreover, almost a third of patients in the step-up approach could successfully avoid surgical necrosectomy. The occurrence of incisional hernia, diabetes mellitus and need for pancreatic enzymes at 6 months was higher in the open necrosectomy group suggesting benefit with use of minimally invasive approach.[25] In another study of 130 patients with infected pancreatic necrosis treated with catheter drainage (predominantly percutaneous), success was achieved in 45 (35%) patients. The factors which suggested failure of PCD in this study were male gender, presence of multiple organ failure, amount of pancreatic necrosis and the morphology of collection on imaging.[26] Another report studied 35 patients with AP who underwent PCD of whom only 13 had infected pancreatic necrosis. Six patients of those with infected necrosis improved with PCD alone, 7 needed surgery while one died. In contrast, of the 22 patients with sterile necrosis 11 improved with PCD alone, 6 needed surgery and 5 died. The difference is possibly related to higher number of patients having multi-organ failure in the sterile necrosis group.[24] In another large study of 60 patients who underwent PCD, the major clinical indication was sepsis in 38 (63%) patients followed by pain in 20 (33%) and biliary obstruction in 2 patients. Overall success was achieved in 54 (90%) patients and three patients needed surgery whereas three patients died.[27]
Multiple systematic reviews have addressed the issue of use of PCD in the management of infected pancreatic necrosis. A recent systematic review included 15 articles reporting data on 577 patients of whom 352 patients had evidence of organ failure before insertion of PCD. Clinical success of PCD was defined as improvement without any further surgical intervention and was achieved in 56.2% (324) patients.[28] While 38.5% of patients needed additional intervention after initial PCD, overall mortality was 18% (104 patients). The major complications recorded were fistulae, organ failure and bleeding. The catheter size used in various studies was variable (6-32 Fr), and some studies reported upsizing of catheters and need for catheter changes. A Cochrane review of interventions in ANP suggested that use of step-up approach resulted in reduction of adverse events and lower costs vis-à-vis open necrosectomy.[29] Many other studies have also reported about the efficacy of PCD as a part of step-up approach in management of infected pancreatic necrosis
Predictors of clinical success for PCD in ANC
Some studies have attempted to identify predictors of success of PCD for infected pancreatic necrosis. In a report of 286 patients with AP who needed intervention, 51 were treated initially with PCD whereas remaining 235 patients underwent surgery. Of the 51 patients initially treated with PCD, 16 (31%) further needed surgery as part of step up approach and the only predictor of failure of PCD was computed tomographic (CT) density of the fluid collection.[30] In a retrospective report of 80 patients with severe AP who underwent placement of PCD, 58 patients had infected ANC. While the CT severity index (CTSI) and modified CTSI (MCTSI) scores did not predict mortality, the CT findings of a higher number of areas of necrosis in the pancreas, presence of distant fluid collections, and posterior para-renal space collections were associated with an increased risk of mortality.[31] The other factors that have been reported by various authors that may predict failure of PCD are enumerated in Table 3. These include organ failure, multiple infected collections and multi-organ infections, amongst others.[26,30,32,33] The importance in recognizing patients who are likely to fail PCD is to timely institute more invasive treatment options in these patients.
 Impact of various technical parameters of PCD placement on outcome
The technical parameters of PCD placement vary in different reports and it is not clear if these viz PCD size, number and location have any impact on the outcome of PCD in ANP. In a retrospective analysis of 80 patients with infected necrosis who received PCD, the outcome was not affected by the initial size of PCD.[34] However, it is expected that a larger bore of PCD may provide a better drainage of the infected material albeit at a heightened risk of pain and other complications. Further prospective randomized studies arerequired to clarify this.
Impact of various technical parameters of PCD placement on outcome
The technical parameters of PCD placement vary in different reports and it is not clear if these viz PCD size, number and location have any impact on the outcome of PCD in ANP. In a retrospective analysis of 80 patients with infected necrosis who received PCD, the outcome was not affected by the initial size of PCD.[34] However, it is expected that a larger bore of PCD may provide a better drainage of the infected material albeit at a heightened risk of pain and other complications. Further prospective randomized studies arerequired to clarify this.
Variations in use of PCD in acute pancreatitis
The available literature shows that the placement of PCD is not according to any standardardized protocol. Various studies report differing indications, methods (ultrasound or CT guided), PCD size (diameter), differences in pattern of management (such as antibiotic prophylaxis), use as part of step-up approach or as an additional armamentarium to endoscopic/surgical drainage. In a report of 663 patients with AP, 122 had moderately severe or severe AP. Of these 122 patients, 47 (39%) underwent PCD. The indications for PCD were non-resolving or enlarging pancreatic fluid collection, presence of systemic inflammatory response syndrome (SIRS) or organ failure, hyper-amylasemia, and worsening inflammatory markers. Antibiotics were not initially given in these patients, as infection was not the primary reason for PCD. No mortality was reported and the predictors of long term PCD requirement were presence of persistent organ failure (POF), higher CTSI, prolonged drainage of amylase rich fluid, or poly-microbial growth in the culture.[35] In another report on PCD for pancreatic collections for uncontrolled pancreatic juice leakage (rather than infected necrosis), 39 out of 56 patients with ANP were drained percutaneously. The major indications for drainage in this report were abdominal pain, inability to resume oral intake, enlarging collections apart from other indication reported in the previous study by Sugimoto. Since infected necrosis was not a pre-requisite for draining collections the drainage was done earlier in the course of the illness vis-à-vis the PANTER trial. The need for necrosectomy, major complication rates and mortality were lower than PANTER trial.[36] Since the comparison is not head to head, the interpretation by the authors that early drainage may be improve outcomes needs to be confirmed by future comparative studies.
 Modifications in percutaneous drainage to improve outcome
Local irrigation: Saline, Antibiotics and Drugs
The saline lavage of the pancreatic collections that have been drained using percutaneous catheters has been reported in a study of 63 patients with infected pancreatic necrosis. Of these, one patients needed open debridement for rapid deterioration, 11 patients needed laparotomy, and five patients died while the strategy succeed in 47 (75%) patients.[37] As the local concentration of the antibiotics attained after systemic administration is a concern in patients with infected pancreatic necrosis, local infusion of antibiotics using nasocystic or percutaneous drainage catheters may be appropriate . In a mathematical model it was suggested that imipenem and piperacillin were likely to be beneficial in local administration while linezolid and tigecycline may not be Debridement
Use of peel-away catheter and use of stone retrieval baskets has been reported to remove necrotic debris through the percutaneous tract. This resulted in avoidance of any need for surgical necrosectomy in 20 patients with infected pancreatic necrosis treated in this manner.[43] Other reports have also used stone retrieval baskets alongside suction catheters for management of infected necrosis.[37] The results however need replication and since the procedure is blind, possibility of injury to adjacent organs and bleeding cannot be entirely avoided.
Modified step-up approach
A paper from China advocated abdominal paracentesis prior to undertaking PCD in all patients who had any amount of drainable fluid present in abdomen. The premise for this approach, termed as a modified step-up approach, is not entirely clear and it may be associated with a risk of introduction of infection into a hitherto sterile collection, although the investigators stated that the risk of infection increases with liquefaction and therefore it is better to drain any fluid before the necrotic debris liquefy. Of the 120 patients with moderately severe or severe AP, 40 patients received abdominal paracentesis alone while 52 needed additional PCD and 14 of these needed necrosectomy.[44] In another paper by the same group, a retrospective analysis suggested abdominal paracentesis seemed to improve outcome parameters including mortality, hospital stay, and need for further intervention. The authors also reported that this improvement was also related to a greater reduction in the serum levels of lipids including triglycerides and free fatty acid in the paracentesis group.[45]
The authors have also demonstrated that paracentesis may not heighten the risk of development of infection of the pancreatic necrotic collections and on the contrary may reduce it.[46] However, in absence of randomized controlled trials from other centers demonstrating the benefits of this alternative step-up approach, its use cannot be recommended at present. Another paper reports about a sequential step-up approach, which included four sequential phases: PCD, mini-incision drainage (MID), video assisted debridement and surgery.[47]
Multi-modality drainage
PCD drainage can also be used as part of a multimodality approach in combination with endoscopic approaches. In a report of 117 patients with symptomatic WOPN, the combined use of endoscopic and percutaneous drainage was reported as part of a dual-modality drainage (DMD). In this subset no surgery was needed and 4 patients (3.4%) eventually died.[48] However, endoscopic drainage is feasible only if the collections have developed a wall and may not be appropriate in patients with early collections. However, when feasible, DMD may reduce length of hospitalization, radiological procedures and number of endoscopies in these patients.[49]
A recent paper had attempted a dual drainage in early pancreatitis by using a trans-papillary (instead of transmural) approach as the second head of drainage in addition to percutaneous drainage. This approach is, therefore, feasible in pancreatic fluid collections which have not yet developed a wall. There was a reduced requirement of surgical intervention and fistula closure was successful in 12 of the 13 patients having infected fluid collection and fistula.[50] After the creation of the internal-external communication, lavage with high volume saline irrigation using 500-2000 ml of saline daily has been effective in 7 patients where surgery could be avoided.[51] Of note, similar approaches to achieve better drainage of PFCs have also been reported with endoscopic drainage, termed the multi-gate drainage technique.[52,53]
Conclusion
Percutaneous drainage of pancreatic collections has emerged as an important measure to manage infected pancreatic necrosis as the initial step of the step-up approach and allows temporization of sepsis and organ failure in a substantial number of patients and helps avoid a morbid procedure in the form of necrosectomy. Apart from infected necrosis, this approach may find utility in management of a subset of patients with sterile collections and walled off collections. However, further refinements and innovations are required to improve the results of percutaneous interventions in acute necrotizing pancreatitis especially in collections with significant amount of solid necrotic material.
References
Modifications in percutaneous drainage to improve outcome
Local irrigation: Saline, Antibiotics and Drugs
The saline lavage of the pancreatic collections that have been drained using percutaneous catheters has been reported in a study of 63 patients with infected pancreatic necrosis. Of these, one patients needed open debridement for rapid deterioration, 11 patients needed laparotomy, and five patients died while the strategy succeed in 47 (75%) patients.[37] As the local concentration of the antibiotics attained after systemic administration is a concern in patients with infected pancreatic necrosis, local infusion of antibiotics using nasocystic or percutaneous drainage catheters may be appropriate . In a mathematical model it was suggested that imipenem and piperacillin were likely to be beneficial in local administration while linezolid and tigecycline may not be Debridement
Use of peel-away catheter and use of stone retrieval baskets has been reported to remove necrotic debris through the percutaneous tract. This resulted in avoidance of any need for surgical necrosectomy in 20 patients with infected pancreatic necrosis treated in this manner.[43] Other reports have also used stone retrieval baskets alongside suction catheters for management of infected necrosis.[37] The results however need replication and since the procedure is blind, possibility of injury to adjacent organs and bleeding cannot be entirely avoided.
Modified step-up approach
A paper from China advocated abdominal paracentesis prior to undertaking PCD in all patients who had any amount of drainable fluid present in abdomen. The premise for this approach, termed as a modified step-up approach, is not entirely clear and it may be associated with a risk of introduction of infection into a hitherto sterile collection, although the investigators stated that the risk of infection increases with liquefaction and therefore it is better to drain any fluid before the necrotic debris liquefy. Of the 120 patients with moderately severe or severe AP, 40 patients received abdominal paracentesis alone while 52 needed additional PCD and 14 of these needed necrosectomy.[44] In another paper by the same group, a retrospective analysis suggested abdominal paracentesis seemed to improve outcome parameters including mortality, hospital stay, and need for further intervention. The authors also reported that this improvement was also related to a greater reduction in the serum levels of lipids including triglycerides and free fatty acid in the paracentesis group.[45]
The authors have also demonstrated that paracentesis may not heighten the risk of development of infection of the pancreatic necrotic collections and on the contrary may reduce it.[46] However, in absence of randomized controlled trials from other centers demonstrating the benefits of this alternative step-up approach, its use cannot be recommended at present. Another paper reports about a sequential step-up approach, which included four sequential phases: PCD, mini-incision drainage (MID), video assisted debridement and surgery.[47]
Multi-modality drainage
PCD drainage can also be used as part of a multimodality approach in combination with endoscopic approaches. In a report of 117 patients with symptomatic WOPN, the combined use of endoscopic and percutaneous drainage was reported as part of a dual-modality drainage (DMD). In this subset no surgery was needed and 4 patients (3.4%) eventually died.[48] However, endoscopic drainage is feasible only if the collections have developed a wall and may not be appropriate in patients with early collections. However, when feasible, DMD may reduce length of hospitalization, radiological procedures and number of endoscopies in these patients.[49]
A recent paper had attempted a dual drainage in early pancreatitis by using a trans-papillary (instead of transmural) approach as the second head of drainage in addition to percutaneous drainage. This approach is, therefore, feasible in pancreatic fluid collections which have not yet developed a wall. There was a reduced requirement of surgical intervention and fistula closure was successful in 12 of the 13 patients having infected fluid collection and fistula.[50] After the creation of the internal-external communication, lavage with high volume saline irrigation using 500-2000 ml of saline daily has been effective in 7 patients where surgery could be avoided.[51] Of note, similar approaches to achieve better drainage of PFCs have also been reported with endoscopic drainage, termed the multi-gate drainage technique.[52,53]
Conclusion
Percutaneous drainage of pancreatic collections has emerged as an important measure to manage infected pancreatic necrosis as the initial step of the step-up approach and allows temporization of sepsis and organ failure in a substantial number of patients and helps avoid a morbid procedure in the form of necrosectomy. Apart from infected necrosis, this approach may find utility in management of a subset of patients with sterile collections and walled off collections. However, further refinements and innovations are required to improve the results of percutaneous interventions in acute necrotizing pancreatitis especially in collections with significant amount of solid necrotic material.
References
- Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11.
- Sharma V, Rana SS, Bhasin DK. Extra-pancreatic necrosis alone: Contours of an emerging entity. JGastroenterol Hepatol. 2016;31:1414-21.
- Rana SS, Sharma V, Sharma RK, et al. Clinical significance of presence and extent of extrapancreatic necrosis in acute pancreatitis. J Gastroenterol Hepatol. 2015;30:794-8.
- Rana SS, Bhasin DK, Reddy Y, et al. Morphological features of fluid collections on endoscopic ultrasound in acute necrotizing pancreatitis: do they change over time? Ann Gastroenterol. 2014;27:258-261.
- Rana SS, Bhasin DK, Sharma RK, et al. Do the morphological features of walled off pancreatic necrosis on endoscopic ultrasound determine the outcome of endoscopic transmural drainage? Endosc Ultrasound. 2014 ;3:118-22.
- Peng T, Dong LM, Zhao X, et al. Minimally invasive percutaneous catheter drainage versus open laparotomy with temporary closure for treatment of abdominal compartment syndrome in patients with early-stage severe acute pancreatitis. J Huazhong Univ Sci Technolog Med Sci. 2016;36:99-105.
- Sharma V, Rana SS, Bhasin DK. Endoscopic ultrasound guided interventional procedures. World J Gastrointest Endosc. 2015;7:628-42.
- Rana SS, Bhasin DK. Non-fluoroscopic endoscopic ultrasound-guided transmural drainage of pseudocysts: A pictorial technical review. Endosc Ultrasound. 2015;4:92-7.
- Soliani P, Franzini C, Ziegler S, et al. Pancreatic pseudocysts following acute pancreatitis: risk factors influencing therapeutic outcomes. JOP 2004;5:38-47.
- Heider R, Meyer AA, Galanko JA ,et al. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229:781-7.
- Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215:571-6.
- Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg. 2002;235:751-8.
- Pelizzari E, Lovera M, Pirrotta AA. Simple and infected pancreatic pseudocysts. Methods of ultrasonography-guided percutaneous needle aspiration: results. Minerva Chir. 1998;53:593-8.
- Mathers B, Moyer M, Mathew A, et al.. Percutaneous debridement and washout of walled-off abdominal abscess and necrosis using flexible endoscopy: a large single-center experience. EndoscInt Open. 2016;4:E102-6.
- Dhingra R, Srivastava S, Behra S, et al. Single or multiport percutaneous endoscopic necrosectomy performed with the patient under conscious sedation is a safe and effective treatment for infected pancreatic necrosis (with video).Gastrointest Endosc. 2015; 81:351-9.
- Mui LM, Wong SK, Ng EK, et al.. Combined sinus tract endoscopy and endoscopic retrograde cholangiopancreatography in management of pancreatic necrosis and abscess. Surg Endosc. 2005;19:393-7.
- Kumar N, Conwell DL, Thompson CC. Direct endoscopic necrosectomy versus step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43:1334-9.
- Chen GY, Dai RW, Luo H, et al.. Effect of percutaneous catheter drainage on pancreatic injury in rats with severe acute pancreatitis induced by sodium taurocholate. Pancreatology. 2015;15:71-7.
- Kotán R, Sápy P, Sipka S, et al. Serum C-reactive protein and white blood cell level as markers of successful percutaneous drainage of acute sterile peripancreatic fluid collection. Chirurgia (Bucur) 2015;110:56-9.
- Walser EM, Nealon WH, Marroquin S, et al. J. Sterile fluid collections in acute pancreatitis: catheter drainage versus simple aspiration. Cardiovasc Intervent Radiol. 2006;29:102-7.
- Zerem E, Imamovic G, Omerovic S, et al.. Randomized controlled trial on sterile fluid collections management in acute pancreatitis: should they be removed? Surg Endosc. 2009;23:2770-7.
- Freeman ML, Werner J, van Santvoort HC, et al. International Multidisciplinary Panel of Speakers and Moderators. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176-94.
- Wang T, Liu LY, Luo H, et al. Intra-Abdominal Pressure Reduction After Percutaneous Catheter Drainage Is a Protective Factor for Severe Pancreatitis Patients with Sterile Fluid Collections. Pancreas. 2016;45:127-33.
- Mortelé KJ, Girshman J, Szejnfeld D, et al. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009;192:110-6.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-502.
- Hollemans RA, Bollen TL, van Brunschot S, et al; Dutch Pancreatitis Study Group. Predicting Success of Catheter Drainage in Infected Necrotizing Pancreatitis. Ann Surg. 2016;263:787-92.
- Fotoohi M, D’Agostino HB, Wollman B, et al.Persistent pancreatocutaneous fistula after percutaneous drainage of pancreatic fluid collections: role of cause and severity of pancreatitis. Radiology 1999;213:573-8.
- Ke L, Li J, Hu P ,et al. Percutaneous Catheter Drainage in Infected Pancreatitis Necrosis: a Systematic Review. Indian J Surg. 2016;78:221-8.
- Gurusamy KS, Belgaumkar AP, Haswell A , et al. Interventions for necrotising pancreatitis. Cochrane Database Syst Rev. 2016;4:CD011383.
- Guo Q, Li A, Hu W. Predictive factors for successful ultrasound-guided percutaneous drainage in necrotizing pancreatitis. SurgEndosc2016;30:2929-34.
- Heiss P, Bruennler T, Salzberger B , et al. Severe acute pancreatitis requiring drainage therapy: findings on computed tomography as predictor of patient outcome. Pancreatology.2010;10:726-33
- Li A, Cao F, Li J , et al. Step-up mini-invasive surgery for infected pancreatic necrosis: Results from prospective cohort study. Pancreatology. 2016 ;pii: S1424-3903(16)30007-2
- Babu RY, Gupta R, Kang M , et al. Predictors of surgery in patients with severe acute pancreatitis managed by the step-up approach. Ann Surg. 2013;257:737-50.
- Bruennler T, Langgartner J, Lang S, et al. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008;14:725-30.
- Sugimoto M, Sonntag DP, Flint GS, et al. A percutaneous drainage protocol for severe and moderately severe acute pancreatitis. SurgEndosc2015;29:3282-91.'
- Sugimoto M, Sonntag DP, Flint GS, et al. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J Vasc Interv Radiol 2016;27:418-25
- Sleeman D, Levi DM, Cheung MC ,et al. Percutaneous lavage as primary treatment for infected pancreatic necrosis. J Am Coll Surg. 2011;212:748-52
- González-López J, Macías-García F, Lariño-Noia J , et al.. Theoretical approach to local infusion of antibiotics for infected pancreatic necrosis. Pancreatology. 2016. pii: S1424-3903(16)30464-1. doi: 10.1016/j. pan.2016.05.396. [Epub ahead of print]
- Inoue T, Ichikawa H, Okumura F, et al. Local administration of amphotericin B and percutaneous endoscopic necrosectomy for refractory fungal-infected walled-off necrosis: a case report and literature review. Medicine (Baltimore) 2015; 94:e558.
- Zerem E, Omerovic S. Successful percutaneous drainage with iodine irrigation for pancreatic fistulas and abscesses after necrotizing pancreatitis. Med Princ Pract. 2012;21:398-400
- Gupta R, Shenvi SD, Nada R, et al. Streptokinase may play role in expanding non-operative management of infected walled off pancreatic necrosis: preliminary results. Pancreatology. 2014; 14:415-8.
- Tang LJ, Wang T, Cui JF, et al. Percutaneous catheter drainage in combination with choledochoscope-guided debridement in treatment of peripancreatic infection. World J Gastroenterol. 2010;16:513-7.
- Echenique AM, Sleeman D, Yrizarry J, et al. Percutaneous catheter-directed debridement of infected pancreatic necrosis: results in 20 patients. J Vasc Interv Radiol. 1998;9:565-71.
- Liu WH, Wang T, Yan HT, et al. Predictors of percutaneous catheter drainage (PCD) after abdominal paracentesis drainage (APD) in patients with moderately severe or severe acute pancreatitis along with fluid collections. PLoS One 2015;10:e0115348.
- Huang Z, Yu SH, Liang HY, et al. Outcome benefit of abdominal paracentesis drainage for severe acute pancreatitis patients with serum triglyceride elevation by decreasing serum lipid metabolites.Lipids Health Dis. 2016;15:110.
- Liu L, Yan H, Liu W, et al. Abdominal Paracentesis Drainage Does Not Increase Infection in Severe Acute Pancreatitis: A Prospective Study. J Clin Gastroenterol. 2015; 49:757-63.
- Li A, Cao F, Li J , et al.. Step-up mini-invasive surgery for infected pancreatic necrosis: Results from prospective cohort study. Pancreatology. 2016 .pii: S1424-3903(16)30007-2. doi: 10.1016/j. pan.2016.03.014. [Epub ahead of print]
- Ross AS, Irani S, Gan SI, et al. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes.Gastrointest Endosc. 2014;79:929-35
- Gluck M, Ross A, Irani S, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg. 2012;16:248-56;
- Yokoi Y, Kikuyama M, Kurokami T , et al. Early dual drainage combining transpapillary endotherapy and percutaneous catheter drainage in patients with pancreatic fistula associated with severe acute pancreatitis. Pancreatology. 2016. pii: S1424-3903(16)00082-X. doi: 10.1016/j.pan.2016.03.007.
- Becker V, Huber W, Meining A, et al. Infected necrosis in severe pancreatitis--combined nonsurgical multi-drainage with directed trans-abdominal high-volume lavage in critically ill patients. Pancreatology. 2009;9:280-6.
- Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc 2011;74:74-80.
- Rana SS, Sharma V, Gorka S , et al.. Creation of multiple transluminal gateway during endoscopic ultrasound-guided drainage of pancreatic necrosis by enlarging tract of impending rupture in duodenum. Endosc Ultrasound. 2015;4:257-9.
- Freeny PC, Hauptmann E, Althaus SJ , et al. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170:969-75.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg. 2000 ;231:361-7.
- Navalho M, Pires F, Duarte A , et al. Percutaneous drainage of infected pancreatic fluid collections in critically ill patients: correlation with C-reactive protein values. Clin Imaging. 2006 ;30:114-9.
- Zerem E, Imamovic G, Sušic A , et al. Step-up approach to infected necrotising pancreatitis: a 20-year experience of percutaneous drainage in a single centre. Dig Liver Dis 2011;43:478-83.
- Baudin G, Chassang M, Gelsi E, et al. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR Am J Roentgenol2012;199:192-9.
|