Wani Bhushan N, Jajoo Suhas N
Department of Surgery,
DMIMSU Jawaharlal Nehru Medical CollegeSawangi (Meghe),
Wardha 442004
Maharashtra, India.
Corresponding Author:
Dr Bhushan Wani
Email: drbnwani@yahoo.co.in
Abstract
The causes of jaundice in the first few weeks of life may be categorised into hematologic,enzymatic/metabolic, infectious and obstructive. Obstructive jaundice results from aninterruption in the drainage of bile in the biliary system. Surgical causes of jaundice inneonates are biliary atresia, inspissated bile syndrome, intrahepatic hypoplasia, choledochalcyst, Caroli’s disease and spontaneous perforation of the bile duct. Pediatricians should beaware of the pernicious consequences of unresolved biliary obstruction and should thusrefer neonates or infants with inexplicable jaundice for surgical exploration at an earlier age.
|
48uep6bbphidvals|168 48uep6bbphidcol4|ID 48uep6bbph|2000F98CTab_Articles|Fulltext The causes of jaundice in the first weeks of life can becategorised into hematologic, enzymatic/metabolic, infectiousand obstructive.[1,2] Diagnostic tests are definitive and surgicaltreatment options do not have a role in the first threecategories. Operative cholangiography is the next logical stepin the diagnosis of obstructive causes.[2,3] Obstructive jaundiceis caused by an interruption to the drainage of bile in the biliarysystem. Surgical causes of jaundice in neonates are biliaryatresia, inspissated bile syndrome, intrahepatic hypoplasia,choledochal cyst, Caroli’s disease and spontaneousperforation of the bile duct.[1,2,3]
Biliary atresia (BA)It is a pathologic process leading to fibrosis and obliterationof the ductal system resulting in obstruction to bile flow, andcholestasis. The aetiology of this entity is unknown. Theincidence of BA is high in China and Japan (1:8,000) ascompared to Europe (1:17,000 live births), and is morecommon in girls than in boys.[4,5,6] A higher incidence is found ininfants less than 32 weeks of gestational age, where thematernal age at conception is more than 35 years, and inmothers with parity 4 or more.[7]
Aetiopathogenesis
Evidence suggests that a few cases related to abnormalmorphogenesis of the bile ducts occur early in gestation, whilstothers appear to arise from damage to the normally developingbile ducts.[4,5,6,7,8] Desmet’s theory suggests the interruption of thenormal remodelling process of the primitive ductal plate. Local ischemia during foetal hepatobiliary development turns intoflow obstruction. As per Howard’s hypothesis, bile leakagefrom abnormal ducts causes inflammatory reaction withsubsequent obliteration of the biliary tree.[9]
During pregnancy, exposure to environmental toxins leadsto infantile obstructive cholangiopathy. Some commonlyinvolved viruses, reovirus-3, cytomegalovirus, rotavirus C,human papilloma virus and retroviruses have been reportedin the literature.[1,2,3,9] Obstruction to the flow of bile causesprogressive cholestasis due to toxic, hydrophobic bile acids.BA may be an acquired rather than a hereditary disease asdiscordance has been seen in monozygotic twins.[10,11,12,13,14,15] Approximately 10% of all cases have been associated withanomalies such as polysplenia, asplenia, situs inversus,absence of the inferior vena cava and pre-duodenal portalvein, for which the term biliary atresia splenic malformation(BASM) syndrome has been coined in the literature.[3,16,17] Otherassociated anomalies are cleft lip, atresia of esophagus,duodenum, and/or jejunum; annular pancreas, malrotation ofgut, and polycystic kidney.[5,6]
Pathology
In the intralobular spaces of the liver, bile stasis, distortion,focal necrosis, hemosiderin deposition in liver cells andintralobular fibrosis occur.[18] In the interlobular spaces,widening of portal area, hepatic fibrosis, periportal oedema,ductular proliferation and bile stasis are noted.[19] Even aftersurgery, when bile flow is regular, intrahepatic bile ducts arenot restored to normal morphology; although some reductionin hepatic fibrosis and inflammatory cells is seen. So surgery is most effective within 60 days of birth, since the hepatic ductsundergo progressive destruction.[4,20,21]
Classification
Biliary atresia is classified as correctable (20%) with distalbiliary tree fibrosis and patent intrahepatic bile ducts andproximal biliary tree; and non–correctable (80%) with fibrosisupto the level of the porta hepatis. Anatomical classificationbased on the pattern of extrahepatic biliary tract remnant iswidely accepted. (Table 1)[4,5,6]

Clinical features
The neonate may present with a history of normal birth weight,varying degrees of jaundice, clay-coloured stools and darkyellow urine. Failure to thrive, coagulopathy and anaemia arealso common. On examination, hepatomegaly, signs ofadvanced disease and cirrhosis, such as ascites, umbilicalhernia, prominent abdominal veins and respiratory discomfortmay be present.
Biochemical tests
A rise in total bilirubin and fall in proteins (albumin) with reversalof the albumin/globulin ratio is noted in advanced cases. Levelsof alkaline phosphatase and transaminases (ALT, AST) arealso raised. Deranged LFTs correspond to the degree ofparenchymal damage and not to the duration of disease.Hepatitis A, B, and C serology; TORCH titres, alpha-1-antitrypsin, gamma glutamyl transpeptidase (GGPT), andserum lipoprotein-X estimates are carried out to negate thepossibility of other causes of cholestasis.[4]
Ultrasonography
Shrunken, non-distended gallbladder without visible commonduct structure is the usual picture. Triangular cord sign, morethan 4 mm thickness of echogenic anterior wall of the rightportal vein on longitudinal scan, is most indicative of BA.[22,23]This modality may also be used for the differential diagnosisof cholestasis, choledochal cyst, and the polyspleniasyndrome.
Duodenal fluid aspiration test
Duodenal intubation may be carried out for 7-24 hours with aRyle’s tube and the aspirated fluid examined; the presence ofyellow bilirubin pigment, rules out BA.[24] Severe neonatalcholestasis can generate false negative results.
Nuclear imaging
It differentiates obstructive from parenchymal causes ofjaundice. Non-appearance of the isotope in the intestine is specific for biliary atresia in upto 50-75% cases only, becausesevere intrahepatic cholestasis and paucity syndrome mayyield similar results.[25]
Operative cholangiography
The gold standard for the diagnosis of BA and widely employedas a more practical tool is operative cholangiography.[26] Thegallbladder appears small and fibrotic and the dye fails toenter proximally into the hepatobiliary tree.
Percutaneous liver biopsy
This is usually carried out prior to surgery but may be doneperoperatively as well. It has a diagnostic accuracy of 90%.[27] Itreveals portal tract oedema, fibrosis, inflammation, intracellularand canalicular cholestasis, and proliferation of bile ductules.
Management
Currently BA is managed in two phases;[3,4,21] the first phasecomprises the Kasai procedure, which aims to restore bileflow. In the second phase liver transplantation is considered,if bile flow is not restored by the Kasai procedure or life-threatening complications of cirrhosis ensue. In patientspresenting late with advanced cirrhosis, liver transplantationis done as the primary procedure.
Kasai procedure (hepato-porto-enterostomy) has been thestandard for non-correctable BA since 1958.[28] Extended rightsubcostal incision cholangiography and liver biopsy are done.The fibrous remnant of the CBD is dissected beyond theconfluence and the transected liver hilum anastomosed withRoux-en-Y loop of jejunum. Many technical variations arepossible, according to the anatomical pattern of the biliaryremnant.[20,21]
Type 1 :Cholecystoenterostomy, or hepaticoenterostomy
Type 2:Cystoenterostomy where the hilar cystcommunicates with the dystrophic intrahepaticbile ducts, demonstrated on cholangiography
Type 3:Hepatoportocholecystostomy
In portocholecystostomy, the gall bladder is used fordrainage, and requires a patent cystic duct and CBD.Complications such as bile leak, gallbladder obstruction,cholangitis, and kinking of the CBD are more frequent.29-31Liver transplantation is indicated after portoenterostomy. Thefive-year survival after transplantation is 80-90%.[30,31,32]
Postoperatively during the evaluation phase of biliaryatresia, the infant’s diet is typically not altered; breastfeedingis encouraged where possible, but an energeticsupplementation may be required to obtain a 150-180 Kcal/kg/day intake. As long as cholestasis persists,supplementation of fat-soluble vitamins is necessary.Administration of choleretics like ursodeoxycholic acid 20 mg/kg/day has been studied with benefits;[33] prednisolone useremains controversial.[34]
Complications
Cholangitis is the most common complication with anincidence of 40-60% following surgery. It can be reduced bylengthening of 50-70 cm of the roux-en-Y jejunum loop and total diversion of biliary conduit;[35] and construction of theintestinal valve by removing a segment of seromuscular layerof the bowel wall and intussuscepting the denuded mucosaas a nipple.[36] Other complications are portal hypertension,nutritional deficiencies like rickets, pulmonary arteriovenousfistula, ectopic variceal bleed, hepatopulmonary syndrome,pulmonary hypertension and malignancy.
Outcome after successful Kasai operation
If the Kasai operation succeeds in restoring bile flow, theevolution of biliary cirrhosis is prevented or at least delayed,and survival with the native liver has been reported up toadulthood. Factors influencing prognosis are the patient’s ageat the time of surgery, extension of liver fibrosis at surgery,degree of intrahepatic bile duct injury, number of episodes ofascending cholangitis, the surgeon’s expertise and the site ofbile duct obstruction.[30,31,32,37,38]
Choledochal cysts
These are congenital conditions associated with benign cysticdilatation of the bile ducts. Its incidence, although as high as1:1000 in the Asian (Japan) population, is only 1:150 000 inthe West.[3] They are more frequently seen in female casesthan in male cases. About 60% are diagnosed during the firstdecade of life, whilst 20% remain undiagnosed until adulthood.
The preponderance in Asia suggests a role for eithergenetic or environmental factors. Anomaly of thepancreaticobiliary junction results in a long common channeland reflux of pancreatic enzymes into the CBD, leading tomucosal breakdown and dilatation.[39] This can present early(in children) with high grade reflux or later (adulthood) with lowgrade reflux.[40,41]
There can be abnormal autonomic innervation of theextrahepatic biliary tree and weakening of the duct wall due toincreased proximal pressure, because of distal obstruction,which is also responsible for the formation of choledochalcysts.[42,43]
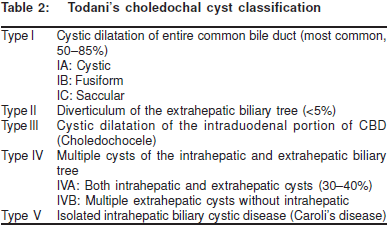
The traditional classification system devised by Alonso-Lej[44] exclusively involved the extrahepatic duct. The clinicalclassification was revised in 1977 by Todani and colleagues.(Table 2)[45]
The patient usually presents with a classical triad ofabdominal pain, cholestatic jaundice (80%), and abdominalmass (30%).2 In patients over 2 years of age, abdominal painis the most common presenting symptom. Bile and pancreaticjuice reflux and bile stasis lead to chronic inflammation, stone and stricture formation. This in turn leads to recurrentcholangitis, hepatic abscesses and pancreatitis, resulting insignificant pain and jaundice.[46,47,48,49,50,51,52] The main diagnostic tool fordetection of a choledochal cyst, especially in childhood, isultrasonography. In adults, computed tomography can confirmthe diagnosis; however, endoscopic retrogradecholangiography or magnetic resonance cholangiography, areconsidered the gold standard.[53]
Surgery is the treatment of choice;[2] excision of the cyst andreconstruction of the biliary tree by choledochal/hepatojejunostomy with a roux-en-Y loop is the standardprocedure for types I and II.[54,55,56] Type III cysts 3 cm or less insize are managed with endoscopic sphincterotomy and cystsmore than 3 cm in size require surgical excision withtransduodenal sphincteroplasty. In Type IVA cysts, for theextraphepatic component, excision and hepaticojejunostomyis done; however, for the intrahepatic component hepaticresection with hepaticojejunostomy is the treatment of choice.Transhepatic intubation may be used for intrahepatic cysts. InType IVB cysts, excision of the cyst with hepaticojejunostomyand transduodenal sphincteroplasty is carried out. For Type Vcysts restricted to one lobe, hepatic resection and livertransplantation are preferred. Bilobar disease represents anespecially challenging problem. Roux-en-Y intrahepaticcholangiojejunostomy or transhepatic silastic intubation for6-12 months may be indicated to improve biliary drainage.
12 months may be indicated to improve biliary drainage.Complications are commonly observed with types I, IV,and V, the overall morbidity rate is less than 10%. Post-surgicalcomplications include cholangitis, biliary stones, anastomoticstricture, residual debris, pancreatitis, and intrahepatic bileduct dilatation.[54] It is important to operate on cholodochal cystsas there is a 20- to 30-fold higher risk in these patients thanthe general population for cholangiocarcinoma. Completeexcision of these lesions is recommended as soon aspossible, preferably before puberty, in order to decrease thechance of developing cancer; the risk remains high even aftersurgery.[57]
Caroli’s Disease
In 1958 Caroli[58] described a congenital anomaly of the biliarytree characterised by multiple cystic dilatations of theintrahepatic bile ducts. In infants, almost all cases of congenitalintrahepatic bile duct dilatation appear to be associated withthe choledochal cyst. The intra-hepatic component isfrequently asymmetrical, involving predominantly one majorintrahepatic branch. Neonates present with jaundice in theearly period which is indistinguishable from presentation ofthe simple choledochal cyst. Ultrasound, CT scan,percutaneous transhepatic cholangiography, endoscopiccholangiopancreatography and magnetic resonancecholangiopancreatography are performed to establish thediagnosis.[59,60,61]
Definitive surgery for most patients is impossible sinceadequate drainage of intrahepatic cysts is difficult to achieve.At the moment, hepatic resection, liver transplantation androux-en-Y intrahepatic cholangiojejunostomy at the liver hilumprovide the best chance for unimpeded bile flow.[61]Transhepatic intubation is recommended as internal drainageis associated with frequent postoperative complicationslike recurrent cholangitis, biliary lithiasis, and hepaticabscess. Malignant degeneration has also beenreported.[62]
Inspissated bile plug
There is mechanical obstruction of the extrahepatic bile ductsby inspissated bile.[63] Inspissated bile syndrome, a misnomerfor a common sequel of erythroblastosis fetalis, results frommassive hemolysis due to Rh or ABO blood groupincompatibility.[64] Obstruction is secondary, as a consequenceof the earlier excessive bile pigment excretory load; it mayproceed to the production of bile pigment stones.[65]
There is persistent jaundice in newborns with hemolyticanaemia, with elevation of both direct and indirect bilirubincomponents. Operative cholangiography is required fordiagnosis. Simple irrigation of the extrahepatic bile ducts iscurative. If choledocholithiasis is present, manual extractionof biliary stones or duodenotomy and sphincterotomy may bedone.[66] The incidence has fallen due to early diagnosis ofblood group incompatibility and prompt exchangetransfusion.[63]
Intrahepatic hypoplasia
Biliary hypoplasia is not a discrete clinical entity; it is anoperative or radiographic finding found in a variety ofhepatobiliary disorders. It is a rare cause of neonatal jaundice.[2,3,4] Affected children have absent or reduced bile ductules withnormal branche distributions of the portal vein and hepaticartery within the liver parenchyma. Biliary hypoplasia is alsoidentified as paucity of interlobular bile ducts (PILBD) and twotypes have been described.
Syndromic biliary hypoplasia (Alagille’s syndrome) ischaracterised by hyperbilirubinemia, characteristic facialappearance, pulmonic artery stenosis, vertebral anomalies,embryotoxon and delayed weight-height development.[67] Non-syndromic biliary hypoplasia is usually clinicallyindistinguishable from biliary atresia. Cholangiography andliver biopsy show diminutive intra- and extra-hepatic biliarytree. However, management is conservative and includespredigested formulae, ursodeoxycholic acids, phenobarbitone,and vitamin A, D, K, E replacement. PILBD has better long-term prognosis.[68]
Spontaneous perforation of the bile duct (SPBD)
Though uncommon, it is potentially fatal and occurs withsufficient frequency to be considered in the differentialdiagnosis of neonatal jaundice.[69] Predisposing factors forperforation are portal bacteremia, stone disease, protein plugs,distal atresia and viral gastroenteritis. Sites of perforation arethe cystic duct, common hepatic duct, and the CBD; thecommonest site is at the junction of the cystic and hepaticducts.[70,71] The perforation is most often pinhole in size,consequently, bile extravasation is gradual, permitting theformation of a biliary pseudocyst. Jaundice is the usual clinicalmanifestation of the disease which becomes apparent duringthe first three months of life. Systemic signs may be minimaland thus the infant easily blends into the diagnostic mix ofsurgical jaundice in infancy.[72]
Sonography shows either generalised ascitis or localisedcollection of fluid, the biliary tree is usually not dilated.Scintigraphy and abdominal paracentesis demonstrate thatthe intraperitoneal fluid originates from the biliary tract.Intraoperative cholangiography is carried out for confirmationof diagnosis at surgery. It can be misinterpreted as a choledochal cyst, which is disastrous if an intestinalanastomosis is performed on the pseudocyst.
Simple peritoneal drainage (percutaneous tube drainage)of the area of ductal perforation is sufficient in the majority ofcases.[73,74] Peritoneal drainage is followed by spontaneousclosure of the perforation, resolution of the biliary pseudocystand cure of the “mechanical obstruction” (probably bile sludge)of the distal common duct. Definitive treatment involves surgery,and repair of perforation.[69,74] Since the perforation of thecommon duct appears to be the only fault in biliaryembryogenesis, the long term prognosis is excellent.
Iatrogenic
Rarely some cases have been reported in the literature ofneonatal obstructive jaundice caused by a malpositionedgastrostomy tube (Foley’s catheter).[75,76,77]
Conclusion
Pediatricians should be aware of the perniciousconsequences of unresolved biliary obstruction and shouldthus refer neonates or infants with inexplicable jaundice forsurgical exploration at an earlier age than was previouslyconsidered appropriate. The specific operations advocatedfor congenital malformation of the biliary tract are 1) Correctablebiliary atresia: roux-en-Y choledochojejunostomy 2) Non-correctable biliary atresia: Kasai’s hepatic portoenterostomyoperation. In the few patients in whom the gallbladder and thedistal biliary tree are still patent, hepatic portocholecystostomyis advocated 3) Billary hypoplasia: Hepatic portoenterostomy4) Choledochal cyst: Total excision andcholedochojejunostomy (roux-en-Y) 5) Caroli’s disease:Resection of the dilated portion of the extra-hepatic bile ductand roux-en-Y choledochojejunostomy. Hepatic resection isdone for intrahepatic cystic disease limited to a single segmentor lobe 6) Inspissated bile plug: Irrigation of the extrahepaticbile ducts and for choledocholithiasis, manual extraction ofbiliary calculi. 7) Spontaneous perforation of the common bileduct: Simple peritoneal drainage at the site of ductal perforationand cholecystostomy.
References:
-
Balistreri WF. Neonatal cholestasis. J Pediatr. 1985;106:171–84.
-
Howard ER. Choledochal cysts. In: Howard ER, (ed) Surgery ofliver disease in children. Oxford: Butterworth-Heinemann;1991:78–90.
-
Davenport M, Betalli P, D’Antiga L, Cheeseman P, Mieli-Vergani G,Howard ER. The spectrum of surgical jaundice in infancy. J PediatrSurg. 2003;38:1471–9.
-
Haber BA, Russo P. Biliary atresia. Gastroenterol Clin North Am.2003;32:891–911.
-
Sinha CK, Davenport M. Biliary atresia. J Indian Assoc PediatrSurg. 2008;13:49–56.
-
Carmi R, Magee CA, Neill CA, Karrer FM. Extrahepatic biliary atresiaand associated anomalies: etiologic heterogeneity suggested bydistinctive patterns of associations. Am J Med Genetics.1993;45:683–93.
-
Fischler B, Haglund B, Hjern A. A population based study on theincidence and possible pre- and perinatal etiologic risk factors ofbiliary atresia. J Pediatr. 2002;141:217–22.
-
Brunero M, De Dreuzy O, Herrera JM, Gauthier F, Valayer L. Prenataldetection of a cyst in the liver hilum. Interpretation for an adequatetreatment. [Article in Italian] Minerva Pediatr. 1996;48:485–94.
-
Bassett MD, Murray KF. Biliary atresia: recent progress. J ClinGastroenterol. 2008;42:720–9.
-
Gunasekaran TS, Hassall EG, Steinbrecher UP, Yong SL.Recurrence of extrahepatic biliary atresia in two half sibs. Am JMed Genet. 1992;43:592–4.
-
Lachaux A, Descos B, Plauchu H, Wright C, Louis D, Raveau J, etal. Familial extrahepatic biliary atresia. J Pediatr GastroenterolNutr. 1988;7:280–3.
-
Smith BM, Laberge JM, Schreiber R, Weber AM, Blanchard H.Familial biliary atresia in three siblings including twins. J PediatrSurg. 1991;26:1331–3.
-
Silveira TR, Salzano FM, Howard ER, Mowat AP. Extrahepaticbiliary atresia and twinning. Braz J Med Biol Res.1991;24:67–71.
-
Hyams JS, Glaser JH, Leichtner AM, Morecki R. Discordance forbiliary atresia in two sets of monozygotic twins. J Pediatr.1985;107:420–2.
-
Poovorawan Y, Chongsrisawat V, Tanunytthawongse C,Norapaksunthorn T, Mutirangura A, Chandrakamol B. Extrahepaticbiliary atresia in twins: Zygosity determination by short tandemrepeat loci. J Med Assoc Thai. 1996;79 Suppl 1:119–24.
-
Davenport M, Savage M, Mowat AP, Howard ER. The biliary atresiasplenic malformation syndrome. Surgery. 1993;113:662–8.
-
Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B,Hadziæ N. The biliary atresia splenic malformation: A 28 year singlecenter retrospective review. J Pediatr. 2006;149:393–400.
-
Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis andoutcome of biliary atresia: current concepts. J PediatrGastroenterol Nutr. 2003;37:4–21.
-
Okazaki T, Miyano G, Yamataka A, Kobayashi H, Koga H, Lane GJ,et al . Diagnostic laparoscopy-assisted cholangiography in infantswith prolonged jaundice. Pediatr Surg Int. 2006;22:140–3.
-
Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, GolmardJL, Auvert B. Prognosis of biliary atresia in the era of livertransplantation: French national study from 1986 to 1996. Hepatology. 1999;30:606–11.
-
Gauthier F, Luciani JL, Chardot C, Branchereau S, de Dreuzy O,Lababidi A, et al. Determinants of life span after Kasai operation atthe era of liver transplantation. Tohoku J Exp Med.1997;181:97–107.
-
Kanegawa K, Akasaka Y, Kitamura E, Nishiyama S, Muraji T, NishijimaE, et al. Sonographic Diagnosis of Biliary Atresia in Pediatric PatientsUsing the Triangular Cord Sign Versus Gallbladder Length andContraction. AJR Am J Roentgenol. 2003;181:1387–90.
-
Farrant P, Meire HB, Mieli-Vergani G. Improved diagnosis ofextrahepatic biliary atresia by high frequency ultrasound of thegallbladder. Br J Radiol. 2001;74:952–4.
-
Meisheri IV, Kasat LS, Kumar A, Bahety G, Sawant V, Kothari P.Duodenal intubation and test for bile: a reliable method to rule outbiliary atresia. Pediatr Surg Int. 2002;18:392–5.
-
Gilmour SM, Hershkop M, Reifen R, Gilday D, Roberts EA. Outcomeof hepatobiliary scanning in neonatal hepatitis syndrome. J NuclMed. 1997;38:1279–82.
-
Meyers RL, Book LS, O’Gorman MA, White KW, Jaffe RB, FeolaPG, et al. Percutaneous cholecysto-cholangiography in thediagnosis of obstructive jaundice in infants. J Pediatr Surg.2004;39:16–8.
-
Zerbini MC, Gallucci SD, Maezono R, Ueno CM, Porta G, MaksoudJ, et al. Liver biopsy in neonatal cholestasis: a review on statisticalgrounds. Mod Pathol. 1997;10:793–9.
-
Kasai M, Suzuki S. A new operation for non-correctable biliaryatresia-hepatic portoenterostomy. Shujitsu 1959;13:733–9.
-
Lilly JR. Hepatic portocholecystostomy for biliary atresia. J PediatrSurg. 1979;14:301–4.
-
Freitas L, Gauthier F, Valayer J. Second operation for repair ofbiliary atresia. J Pediatr Surg. 1987;22:857–60.
-
Karrer FM, Lilly JR, Stewart BA, Hall RJ. Biliary atresia registry,1976 to 1989. J Pediatr Surg. 1990;25:1076–80.
-
Shinkai M, Ohhama Y, Take H, Kitagawa N, Kudo H, Mochizuki K, etal. Long-term outcome of children with biliary atresia who werenot transplanted after the Kasai operation: >20-year experience at a children’s hospital. J Pediatr Gastroenterol Nutr.2009;48:443–50.
-
Balistreri WF. Bile acid therapy in pediatric hepatobiliary disease:the role of ursodeoxycholic acid. J Pediatr Gastroenterol Nutr.1997;24:573–89.
-
Muraji T, Higashimoto Y. The improved outlook for biliary atresiawith corticosteroid therapy. J Pediatr Surg. 1997;32:1103–6.
-
Bu LN, Chen HL, Chang CJ, Ni YH, Hsu HY, Lai HS, et al. Prophylacticoral antibiotics in prevention of recurrent cholangitis after the Kasaiportoenterostomy. J Pediatr Surg. 2003;38:590–3.
-
Muraji T, Tsugawa C, Nishijima E, Satoh S, Takamizawa S, Ise K, etal. Surgical management for intractable cholangitis in biliary atresia. J Pediatr Surg. 2002;37:1713–5.
-
Saeki M, Nakano M, Hagane K, Shimizu K. Effectiveness of anintussusceptive antireflux valve to prevent ascending cholangitisafter hepatic portojejunostomy in biliary atresia. J Pediatr Surg.1991;26:800–3.
-
Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis andoutcome of biliary atresia: current concepts. J PediatrGastroenterol Nutr. 2003;37:4–21.
-
Gigot J, Nagorney D, Farnell M, Moir C, Ilstrup D. Bile duct cysts: achanging spectrum of disease. J Hepatobiliary Pancreat Surg.1996;3:405–11.
-
Babbitt DP. Congenital choledochal cysts: new etiological conceptbased on anomalous relationships of the common bile duct andpancreatic bulb. Ann Radiol (Paris). 1969;12:231–40.
-
Jeong IH, Jung YS, Kim H, Kim BW, Kim JW, Hong J, et al. Amylaselevel in extrahepatic bile duct in adult patients with choledochalcyst plus anomalous pancreatico-biliary ductal union. World JGastroenterol. 2005;11:1965–70.
-
Yotsuyanagi S. Contribution to aetiology and pathology of idiopathiccystic dilatation of the common bile duct with report of three cases. Gann: Japanese Journal of Cancer Research. 1936;30:601–752.
-
Davenport M, Basu R. Under pressure: choledochal malformationmanometry. J Pediatr Surg. 2005;40:331–5.
-
Alonso-Lej F, Rever W, Pessagno DJ. Congenital choledochal cyst,with a report of 2, and an analysis of 94 cases. Int Abstr Surg.1959;108:1–30.
-
Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenitalbile duct cysts: Classification, operative procedures, and reviewof thirty-seven cases including cancer arising from choledochalcyst. Am J Surg. 1977;134:263–9.
-
Landing BH. Considerations of the pathogenesis of neonatalhepatitis, biliary atresia and choledochal cyst: the concept ofinfantile obstructive cholangiopathy. Prog Pediatr Surg.1974;6:113–39.
-
Shi LB, Peng SY, Meng XK, Peng CH, Liu YB, Chen XP,, et al.Diagnosis and treatment of congenital Choledochal cyst: 20 years’experience in China. World J Gastroenterol. 2001;7:732–4.
-
Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL.Choledochal cyst disease: a changing pattern of presentation. Ann Surg. 1994;220:644–52.
-
Rattan KN, Magu S, Ratan S, Chaudhary A, Seth A. Choledochalcyst in children: 15 year experience. Ind J Gastroenterol.2005;24:178.
-
Sela-Herman S, Scharschmidt BF. Choledochal cyst, a diseasefor all ages. Lancet. 1996;347:779.
-
Büyükyavuz Y, Ekinci S, Ciftçi AO, Karnak I, Senocak ME, TanyelFC. A retrospective study of choledochal cyst: clinical presentation,diagnosis and treatment. Turk J Pediatr. 2003;45:321–5.
-
de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA,Bosma A, et al. Choledochal cysts: age of presentation, symptoms,and late complications related to Todani’s classification. J PediatrSurg. 2002;37:1568–73.
-
Kim MJ, Han SJ, Yoon CS, Kim JH, Oh JT, Chung KS, et al. Using MRcholangiopancreatography to reveal anomalous pancreaticobiliaryductal union in infants and children with Choledochal cysts. AJRAm J Roentgenol. 2002;179:209–14.
-
Nagorney D M. Bile duct cysts in adults. In: Blumgart LH, Fong Y,editors. Surgery of the Liver and Biliary Tract and Pancreas. 3rdedition. Saunders, UK; 2001;1229-1244.
-
Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352–9.
-
Metcalfe MS, Wemyss-Holden SA, Maddern GJ. Managementdilemmas with choledochal cysts. Arch Surg. 2003;138:333–9.
-
Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T,Ochiai T. Risk of bile duct carcinogenesis after excision ofextrahepatic bile ducts in pancreaticobiliary maljunction. Surgery.1999;126:939–44.
-
Caroli J, Soupault R, Kossakowski J, Plocker L, Paradowska.Congenital polycystic dilation of the intrahepatic bile ducts; attemptat classification. Sem Hop. 1958;34:488–95/SP.
-
Lopez CA, Munoz BA, Herrera MD, Moreno SO, Flores MM, PerezJJ. Diagnosis of Caroli’s disease with conventionalultrasonography and echo-Doppler. Rev Esp Enferm Dig 1994;85:387–9.
-
Benhidjeb T, Muller JM, Gellert K, Zanow J, Rudolph B. Currenttherapy of bile duct cysts II. Intrahepatic cysts (Caroli syndrome).[Article in German] Chirurg. 1996;67:238–43.
-
Lendoire J, Schelotto PB, Rodríguez JA, Duek F, Quarin C, GarayV, et al. Bile duct cyst type V (Caroli’s disease): surgical strategyand results. HPB (Oxford). 2007;9:281–4.
-
Balsells J, Margarit C, Murio E, Lazaro JL, Charco R, Vidal MT, et al.Adenocarcinoma in Caroli’s disease treated by liver transplantation. HPB Surg. 1993;7:81–6.
-
Kondo M, Kunikata T, Onishi S. Inspissated bile syndrome. [Articlein Japanese] Ryoikibetsu Shokogun Shirizu. 1996;9:524–7.
-
Dunn P. Obstructive jaundice and haemolytic disease of thenewborn. Arch Dis Child 1963;38:596–9.
-
Grosfeld JL, Rescorla FJ, Skinner MA, West KW, Scherer LR 3rd.The spectrum of biliary tract disorders in infants and children.Experience with 300 cases. Arch Surg. 1994;129:513–20.
-
Gunnarsdottir A, Holmqvist P, Arnbjornsson E, Kullendorff CM.Laparoscopic aided cholecystostomy as a treatment of inspissatedbile syndrome. J Pediatr Surg. 2008;43:e33–5.
-
Alagille D, Estrada A, Hadchouel M, Gautier M, Odièvre M,Dommergues JP. Syndromic paucity of interlobular bile ducts(Alagille syndrome or arteriohepatic dysplasia): review of 80cases. J Pediatr. 1987;110:195–200.
-
Kahn E, Daum F, Markowitz J, Teichberg S, Duffy L, Harper R, et al.Nonsyndromatic paucity of interlobular bile ducts: Light and electronmicroscopic evaluation of sequential liver biopsies in earlychildhood. Hepatology. 2005:6;890–901
-
Kasat LS, Borwankar SS, Jain M, Naregal A. Spontaneousperforation of the extrahepatic bile duct in an infant. Pediatr Int.2001;17:463–4.
-
Stringel G, Mercer S. Idiopathic perforation of the biliary tract ininfancy. J Pediatr Surg. 1983;18:546–50.
-
Chardot C, Iskandarani F, De Dreuzy O, Duquesne B, Pariente D,Bernard O, et al. Spontaneous perforation of the biliary tract ininfancy: a series of 11 cases. Eur J Pediatr Surg. 1996;6:341–6.
-
Konoija RP, Shandip KS, Rawat J, Wakhlu A, Kureel S, Tandom R.Spontaneous biliary perforation in infancy and childhood: clues todiagnosis. Indian J Pediatr. 2007;74:509–10.
-
Lilly JR, Weintraub WH, Altman RP. Spontaneous perforation of theextrahepatic bile ducts and bile peritonitis in infancy. Surgery.1974;75:664–73.
-
Prevot J, Babut JM. Spontaneous perforations of the biliary tract ininfancy. Prog Pediatr Surg. 1970;3:187–208.
-
Sebastian MV, Coley BD. Neonatal obstructive jaundice causedby a malpositioned gastrostomy tube. AJR 2005;184:S132–3.
-
Konda J, Ruggle P. Prolapse of Foley catheter gastrostomy tubecausing obstructive jaundice. Am J Gastroenterol.1981;76:353–5.
-
Abu Zidan F, Kumar M, Hassan I, Samhan M, Dadah S. Iatrogenicobstructive jaundice caused by the balloon of a Foley catheter. Clin Nucl Med. 1991;16:757–9.
|