48uep6bbphidvals|139
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Celiac disease is a gluten sensitive enteropathy with a broad spectrum of clinical manifestations ranging from asymptomatic manifestation to typical malabsorption symptoms including diarrhoea, weight loss, anaemia and nutritional deficiencies.(1) Celiac disease is a common cause of chronic diarrhoea and the malabsorption syndrome all over the world. Though it was considered uncommon in India in the past, it has been frequently described recently.(2,3,4)
Most of the patients diagnosed with celiac disease respond to gluten free diet (GFD), with resolution of clinical symptoms and histological and serological recovery.(5) However, some celiac disease patients continue to experience gut symptoms after GFD. In these cases, several pathological conditions ranging from lactose malabsorption to refractory celiac disease may cause the persistence of symptoms.(6) In 2003, Tursi et al described 15 cases of celiac disease unresponsive to GFD in whom small intestinal bacterial overgrowth (SIBO) or lactose intolerance was the reason for no response.(7 )
But the prevalence of SIBO in confirmed cases of celiac patients from North India has not yet been reported. Therefore, the aim of this study was to evaluate the prevalence of small intestinal bacterial overgrowth in patients with celiac disease.
Materials and Methods
We studied 87 patients of confirmed celiac disease (serologically and histologically). All patients enrolled in this study showed a classical form of the disease, with several gastro-intestinal (GI) symptoms (diarrhoea i.e. >3 bowel movements/day, abdominal discomfort / abdominal pain, short stature). Subjects who had received antibiotics(8) and other drugs known to affect gastrointestinal function, in the 2 weeks before the study, were excluded from this analysis. Cigarette smoking(9) and physical excercise sufficient to produce hyperventilation(10) were not allowed during the 2 hours preceding the test.
Glucose hydrogen breath test for small intestinal bacterial overgrowth was done according to the method of Kerlin & Wong.(11) According to this method all subjects were asked to fast from 9 PM on the day before the test and breath samples were obtained at 9 AM in the morning. They were also advised to avoid eating high fibre diet the previous evening, which may cause prolonged excretion of hydrogen.(12) Before obtaining the fasting sample, all subjects were questioned about their compliance with these instructions.
End-expiratory fasting breath samples were collected by a device consisting of a mouthpiece, a T valve and sample collection bag (Quintron Instrument Co., Milwaukee, Wisconsin). The breath samples were then transferred to the sample loop of the chromatograph by a 60 ml syringe. Then the subjects were given 80 g glucose to drink and hydrogen concentration was measured after every 15 minutes in end expiratory breath samples for up to 2 hours. The samples were analysed by Model SC Microlyser from Quintron USA for the hydrogen concentration which was expressed in parts per million (ppm). An increase more than 10 pm over the fasting value within 2 hours was considered positive for small intestinal bacterial overgrowth.
87 age and sex matched apparently healthy controls were also enrolled in this study.
STATISTICAL ANALYSIS
Prevalence of small intestinal bacterial overgrowth in patients and controls was compared by the Student’s t test for unpaired data. All data are expressed as mean ± SD. p value less than 0.05 was taken as significant.
Results
Of 87 patients of celiac disease, 49 (56.3%) were male while 38 (43.7%) were female. The mean (± SD) age for male patients was 26.3 ± 16.3 years (range 14-59 years) and for female patients was 28.4 ± 15.6 years (range 16-58 years). Amongst the 87 controls, 52 (59.8%) were male and 35 (40.2%) were female. The mean(± SD) age for male controls was 27.6 ± 14.5 years (range 15-57 years) and for female controls was 29.3 ± 16.5 years (range 18-59 years).
The prevalence of small intestinal bacterial overgrowth was 20.7% in patients with celiac disease while there was no case of small intestinal bacterial overgrowth in apparently healthy controls (Table I). The difference was statistically significant (p<0.01). The incidence of SIBO in male and female patients was not statistically significant (Table II). The pattern of hydrogen concentration in celiac patients with SIBO positive and negative is depicted in Figure 1. The rise in mean (± SD) values of hydrogen concentration at peak with glucose hydrogen breath test in celiac disease patients with SIBO was 13.5 ± 2.6 ppm. While the rise in mean (± SD) values of hydrogen concentration at peak with glucose hydrogen breath test in celiac disease patients without SIBO was 2.3 ± 1.4 ppm.
Table I. Prevalence of small intestinal bacterial overgrowth (SIBO) in
celiac patients and controls
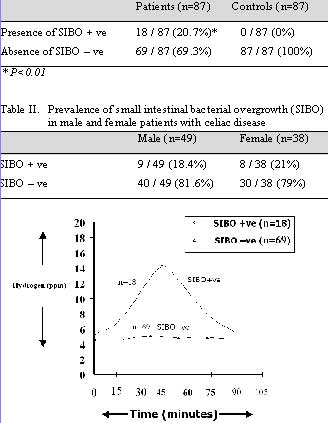
Fig. 1: Pattern of glucose H2BT in celiac patients with and
without SIBO
Discussion
In most celiac patients, gluten free diet (GFD) results in both resolution of clinical symptoms and histological recovery with seroconversion for IgA anti-gliadin and IgA anti-endomysial antibodies and negative sorbitol hydrogen breath test. However, in some rare cases patients complaining of GI symptoms with histological findings consistent with celiac disease are unable to achieve remission of symptoms despite full adherence to GFD, and in these cases other causes of GI symptoms should be considered. In these cases, the first hypothesis may be a dietary mistake, which often occurs despite patients’ adherence to GFD. Another cause may be parasitic infestation. Parasitic infestation (especially Giardia lamblia infestation) should, however, always be excluded before making a diagnosis of celiac disease.
Diagnosis of celiac disease in all the patients was established based on standard criteria.(4) It has been earlier shown that half of the patients with malabsorption resulting from various causes including celiac disease may have SIBO.(13) In patients with tropical sprue it resulted from small intestinal stasis as evidenced by prolonged oro-ceacal transit time (OCTT).(14) Similar mechanisms may operate in patients with MAS due to other causes; the patient with celiac disease with SIBO in this report had prolonged OCTT. Prolonged OCTT has been reported by other workers in patients with celiac disease, which normalised after GFD.(15) Unabsorbed foods within the intestinal lumen may also promote growth of bacteria in the small intestinal lumen. We believe that SIBO in patients with malabsorption due to other causes may have the following clinical significance: 1) response to antibiotics may lead to fallacious diagnosis of tropical sprue as response to antibiotics has been considered an important criterion for diagnosis of this disease(16), 2) it may be a cause for inadequate response or refractory state despite GFD.
Small intestinal bacterial overgrowth in patients with celiac disease may lead to persistent diarrhoea due to disturbances in luminal digestion and alteration of mucosal function. Bacteria in the small intestine of patients with SIBO cause deconjugation of bile acids, which results in watery diarrhoea due to increased colonic secretions and steatorrhoea due to depletion of the bile acid pool.(17) Lactose intolerance results in persistent diarrhoea mainly due to the osmotic effect of unabsorbed lactose, and flatulence due to production of gas from fermentation of unabsorbed lactose.
Conclusion
This study indicates that a significant number of celiac patients have small intestinal bacterial overgrowth. Therefore, all celiac patients should be tested for small intestinal bacterial overgrowth by using the non-invasive glucose hydrogen breath test. Those testing positive for small intestinal bacterial overgrowth should be treated with antibiotics before putting them on a gluten free diet. This may result in complete recovery.
References
1. Tursi A, Giorgetti G, Brandimarte G, Rubino E, Lombardi D, Gasbarrini G. Prevalence and clinical presentation of subclinical/silent celiac disease in adults: an analysis on a 12-year observation. Hepatogastroenterology. 2001;48:462–4.
2. Ghoshal UC, Ghoshal U, Misra A, Choudhuri G. Partially responsive celiac disease resulting from small intestinal bacterial overgrowth and lactose intolerance. BMC Gastroenterol. 2004;4:10.
3. Mohindra S, Yachha SK, Srivastava A, Krishnani N, Aggarwal R, Ghoshal UC, et al. Coeliac disease in Indian children: assessment of clinical, nutritional and pathologic characteristics. J Health Popul Nutr. 2001;19:204–8.
4. Poddar U, Thapa BR, Nain CK, Prasad A, Singh K. Celiac disease in India: Are they true cases of celiac disease? J Pediatr Gastroenterol Nutr. 2002;35:508–512.
5. Trier JS. Celiac sprue. N Engl J Med. 1991;325:1709–19.
6. Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830–8.
7. Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839–43.
8. Gilat T, Ben Hur H, Gelman-Malachi E, Terdiman R, Peled Y. Alterations of the colonic flora and their effect on the hydrogen breath test. Gut. 1978;19:602–5.
9. Thompson DG, Binfield P, De Belder A, O’Brien J, Warren S, Wilson M. Extra intestinal influences on exhaled breath hydrogen measurements during the investigation of gastrointestinal disease. Gut. 1985;26:1349–52.
10. Perman JA, Modler S, Engel RR, Heldt G. Effect of ventilation on breath hydrogen measurements. J Lab Clin Med. 1985;105:436–9.
11. Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–8.
12. Brummer RJ, Armbrecht U, Bosaeus I, Dotevall G, Stockbruegger RW. The hydrogen (H2) breath test. Sampling methods and the influence of dietary fibre on fasting level. Scand J Gastroenterol. 1985;20:1007–13.
13. Ghoshal U, Ghoshal UC, Ranjan P, Naik SR, Ayyagari A. Spectrum and antibiotic sensitivity of bacteria contaminating upper gut in patients with malabsorption syndrome in the tropics. BMC Gastroenterology. 2003;3:9.
14. Ghoshal UC, Ghoshal U, Ayyagari A, Ranjan P, Krishnani N, Misra A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of oro-cecal transit time. J Gastroenterol Hepatol. 2003;18:540–7.
15. Chiarioni G, Bassotti G, Germani U, Battaglia E, Brentegani MT, Morelli A, et al. Gluten-free diet normalizes mouth-to-ceacum transit of a caloric meal in adult patients with celiac disease. Dig Dis Sci. 1997;42:2100–5.
16. Lim ML. A perspective on tropical sprue. Curr Gastroenterol Rep. 2001;3:322–7.
17. Isaacs PE, Kim YS. The contaminated small bowel syndrome. Am J Med. 1979;67:1049–57.