48uep6bbphidvals|137
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Imaging of the liver performed to detect and characterise focal liver lesions has undergone tremendous change in the last decade. While liver ultrasonography (USG) is still the first-choice investigation, the role of CT and MRI has increased manifold in most institutions because of their dynamic and multiphasic imaging capacity. With the introduction of multidetector CT (MDCT) and new robust breath-hold MRI sequences and specific MRI contrast agents during the last decade, the role of MDCT and MRI has changed to a first-step modality in liver lesion characterisation. Advances in contrast-enhanced sonography and 18-fluorodeoxyglucose positron emission tomography (18-FDG-PET) have added a new dimension to functional liver imaging. This article tries to distill the myriad advances in various imaging modalities that are currently in use for liver imaging.
ULTRASONOGRAPHY
Ultrasonography is a cost effective, widely available, noninvasive technique, which provides depth-dependent spatial resolution combined with good signal-to-noise ratio in most pathologic entities of the liver. It plays an important role in initial evaluation of the liver with high detection and specificity rates although it is highly operator and equipment-dependent. In most countries, a combination of USG and serum alfa-fetoprotein levels every 6 months is recommended for screening of hepatocellular carcinoma (HCC) in patients with cirrhosis of liver.(1) Colour Doppler USG aids characterisation of the lesions by demonstration of vascularity but due to its low sensitivity and specificity, other imaging modalities are required for more specific diagnosis.(2) USG has lower lesion detection rates than contrast-enhanced CT or MRI.(3) Attempts have been made to address this lacuna with advances in imaging techniques such as tissue harmonic imaging (THI), real-time spatial compound imaging (Sono-CT), three-dimensional USG, panoramic USG and use of contrast enhanced US (CEUS) (Figure 1).
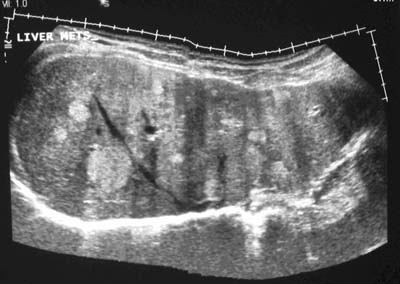
Fig. 1: Panoramic ultrasound shows multiple variable sized hyperechoic metastatic
lesions in the liver.
Three-dimensional USG partially overcomes some of the limitations of conventional USG. It uses multislice function and hence increases the sensitivity of liver tumour detection.(4)It also accurately localises the tumour, provides information of its relation to major vessels and is useful in calculating total volume of liver masses, which is useful in post-chemotherapy follow-up.(4)
Colour Doppler USG is basic imaging of the liver when contrast agents are used. However, its major limitation is the ‘blooming’ artifact. Other contrast specific imaging techniques are used which include second harmonic B-mode imaging, harmonic colour and power Doppler imaging, and the phase inversion technique. Of these the latter is most frequently employed.(5)
With the development of the first-generation microbubble USG contrast agent, dynamic imaging of the liver was introduced into sonography. Contrast enhanced USG entails use of microbubble contrast agents which are gas bubbles 1-10 microns in diameter. The gas could be either air (nitrogen) or perfluorocarbons. On intravenous injection these survive in the vascular pool for several minutes and thus allow imaging of the liver.(2) Microbubble contrast agents increase the intensity of the blood flow signal, improving the detection of flow in the hepatic artery and smaller vessels, and are as effective as CT in assessing lesion perfusion.(6,7) However, first generation agents are usually disrupted by scanning and hence used with intermittent scanning. Second-generation agents, such as Levovist (SHU 580A) is different from others in that it has liver specific uptake and is useful in liver tumour characterisation. It has also been used in the analysis of hepatic transit time.(8) Early enhancement of hepatic veins is seen in cirrhosis and malignancy due to arterialisation and vascular shunting.(9) Analysis of the transit time allows assessment of disease severity, with shorter transit times seen in more severe disease.(10) Late phase imaging can also be done which helps in better differentiation of malignant (seen as uptake abnormality) and benign (showing prolonged uptake) lesions.(11) The late phase imaging done using the phase inversion technique is useful in improving the accuracy of USG guided liver biopsy.(12)
SonoVue (Sulphur hexafluoride), taking advantage of stable phospholipid-coated gas particles, can withstand the acoustic pressure of insonation much better resulting in increased half-time of the agent and thus allowing continuous real-time imaging of contrast enhancement during the arterial (15 seconds), portal (60 seconds) and late phases (2-5 minutes).(13) These agents are used not only to increase the Doppler signal amplitude during the vascular phase, but also to differentiate hepatocellular tissue from neoplastic disease. The selective uptake of these microbubbles by the normal liver parenchyma with probable reticuloendothelial system (RES) interaction during the "late phase" (2-4 minutes after contrast administration), increases the lesion-to-liver contrast. Since metastases are not able to "store" the microbubbles, they appear as hypoechoic "holes" on CEUS. This technique is also useful in detection of small lesions less than 1cm in size where other imaging modalities have lower sensitivity.(3)
With the use of the phase inversion technique, there is increased echogenicity of the normal hepatic parenchyma leading to increased conspicuity of focal liver lesions like metastasis, particularly of the ones that are small in size.(8) The overall performance of CEUS is similar to that of dual phase CT and it can therefore be a competitive alternative in the imaging of hepatic metastasis.(8)
Flash echo contrast sonography (FECS) is an intermittent harmonic imaging technique for visualisation of tissue perfusion. It is more sensitive than power Doppler in determining vascularity of small HCC.(14) FECS shows comparative ability with CT and angiography to detect residual tumour after local ablation of small HCC.(15)
Contrast coherent imaging is a non-linear imaging technology that provides high sensitivity in microbubble detection. It allows access to broadband harmonic image formation and achieves higher frame rate.(16) This technique using Sonovue as the contrast agent helps in better detection of HCC and in assessment of cellular differentiation based on the echogenicity.(16) The use of low-mechanical index technique also improves characterisation of focal liver lesions with high sensitivity and specificity.(17)
These facts have led to marked increase in the use of secondgeneration USG contrast agents in the evaluation of liver in oncology patients. In addition, the dynamic enhancement characteristics of haemangiomas, focal nodular hyperplasia (FNH) and adenoma are similar to that seen on CT and MRI. Of these, FNH is probably best characterised on CEUS showing the central feeding artery and spoke wheel pattern better due to its high spatial and temporal resolution.(5) CEUS is also useful has also been used in the analysis of hepatic transit time.(8) Early enhancement of hepatic veins is seen in cirrhosis and malignancy due to arterialisation and vascular shunting.(9) Analysis of the transit time allows assessment of disease severity, with shorter transit times seen in more severe disease.(10) Late phase imaging can also be done which helps in better differentiation of malignant (seen as uptake abnormality) and benign (showing prolonged uptake) lesions.(11) The late phase imaging done using the phase inversion technique is useful in improving the accuracy of USG guided liver biopsy.(12)
SonoVue (Sulphur hexafluoride), taking advantage of stable phospholipid-coated gas particles, can withstand the acoustic pressure of insonation much better resulting in increased half-time of the agent and thus allowing continuous real-time imaging of contrast enhancement during the arterial (15 seconds), portal (60 seconds) and late phases (2-5 minutes).(13) These agents are used not only to increase the Doppler signal amplitude during the vascular phase, but also to differentiate hepatocellular tissue from neoplastic disease. The selective uptake of these microbubbles by the normal liver parenchyma with probable reticuloendothelial system (RES) interaction during the "late phase" (2-4 minutes after contrast administration), increases the lesion-to-liver contrast. Since metastases are not able to "store" the microbubbles, they appear as hypoechoic "holes" on CEUS. This technique is also useful in detection of small lesions less than 1cm in size where other imaging modalities have lower sensitivity.(3)
With the use of the phase inversion technique, there is increased echogenicity of the normal hepatic parenchyma leading to increased conspicuity of focal liver lesions like metastasis, particularly of the ones that are small in size.(8) The overall performance of CEUS is similar to that of dual phase CT and it can therefore be a competitive alternative in the imaging of hepatic metastasis.(8)
Flash echo contrast sonography (FECS) is an intermittent harmonic imaging technique for visualisation of tissue perfusion. It is more sensitive than power Doppler in determining vascularity of small HCC.(14) FECS shows comparative ability with CT and angiography to detect residual tumour after local ablation of small HCC.(15)
Contrast coherent imaging is a non-linear imaging technology that provides high sensitivity in microbubble detection. It allows access to broadband harmonic image formation and achieves higher frame rate.(16) This technique using Sonovue as the contrast agent helps in better detection of HCC and in assessment of cellular differentiation based on the echogenicity.(16) The use of low-mechanical index technique also improves characterisation of focal liver lesions with high sensitivity and specificity.(17)
These facts have led to marked increase in the use of secondgeneration USG contrast agents in the evaluation of liver in oncology patients. In addition, the dynamic enhancement characteristics of haemangiomas, focal nodular hyperplasia (FNH) and adenoma are similar to that seen on CT and MRI. Of these, FNH is probably best characterised on CEUS showing the central feeding artery and spoke wheel pattern better due to its high spatial and temporal resolution.(5) CEUS is also useful in the evaluation of vascular disease.(18) Furthermore, CEUS is valuable in the monitoring of post radiofrequency ablative procedures for detecting residual tumour, active bleeding or intraparenchymal liver haematomas.(19,20) After transarterial chemoembolisation for HCC, CEUS can be performed within the first two weeks for assessing treatment efficacy, instead of waiting for three months for detection by CT or MRI.(21) The second generation agent SonoVue dramatically increases the diagnostic accuracy leading to sensitivity and specificity values probably very close to CT or MRI.(16,22)
MULTIDETECTOR CT
Multidetector helical CT is the latest inspiration. Its evolution continued through late 1998 with the ability to scan multiple slices simultaneously. The advent of MDCT technology has led to increased temporal and spatial resolution along the zaxis and longer anatomic coverage. Fast data acquisition and breath-hold scanning allows imaging of the liver twice; in the hepatic arterial dominant phase and portal venous phase, prior to the equilibrium phase. Studies have shown that this biphasic approach to scanning has greatly improved the detection of HCC.(23,24) Excellent 3-D data sets can be produced improving multiplanar imaging, which allows evaluation of subcapsular lesions, demonstration of vascular anatomy and better characterisation of the lesions.(25) Together with improvements in bolus-tracking, MDCT scanning during the various vascular contrast and equilibrium phases allows performing CTangiography of the liver and mesenteric vessels which can be important in patients undergoing hepatic resection, liver transplantation and transarterial chemo or radio-embolisation. MDCT portal venogram is useful in evaluation of the portal system along with extent and location of collaterals in portal hypertension.(26) The maximum intensity projections provide a complete picture of complex collaterisation of the portal vein in potential liver transplant recipients. Following lesion detection, dynamic evaluation of the vascularity and microvasculature of liver parenchyma and hepatic neoplasms also aids characterisation of focal liver lesions.(27) With the newer systems, faster scanning is possible with multiple phases obtained in single breath hold; this allows subtraction of pre-contrast from post-contrast images. Additionally quantitative perfusion studies can also be done.(28,29) Thus, MDCT can be used as a “one-stop-shop” in liver imaging for evaluating the liver lesion, liver parenchyma and hepatic vessels in the same sitting. MDCT also brings with it new challenges concerning the acquisition parameters and contrast-injection protocols that should be adjusted to the clinical situation and type of lesion expected.
Foley and Malisee30 proposed a triple-phase technique with MDCT for the detection of hypervascular neoplasms. The first acquisition (early arterial phase done at 15 seconds) provides a true arterial phase with no admixture of enhanced portal venous blood, whereas in the late arterial phase (done after 30 seconds) an initial opacification of the portal venous blood occurs (“portal-venous-inflow” phase). The former is useful for providing angiographic images while the latter is beneficial for the detection of hypervascular tumours (Figure 2a,b).(30) The third acquisition results at 60 seconds after the start of contrast material infusion. In addition a delayed or equilibrium phase is performed at approximately 90-120 seconds. The use of 3 phases is useful for the detection and staging of HCC. Moreover, postablation follow up by MDCT is highly advantageous in patients of HCC for picking up residual disease and fresh lesions, which have an important bearing on patient management (Figure 3). The rate of contrast injection also plays an important role in detection of HCC. An injection rate of 5 ml/ s recovers more lesions when compared with 3 ml/s with no increase in discomfort or side-effects.(31) The diagnostic accuracy of the multiphasic CT is similar to superparamagnetic iron oxide (SPIO) enhanced MRI in terms of sensitivity, specificity and positive predictive value for the detection of HCC.(32,33)
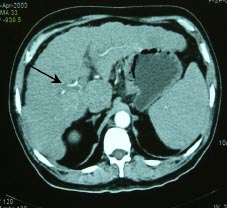
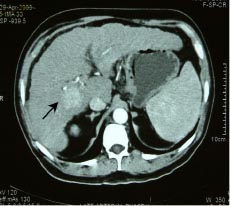
Fig. 2a,b: Multiphasic multi-detector computed tomography (MDCT) of a 68 year old cirrhotic
illustrating the importance of early(a) and late arterial (b) phases by depicting good vascular
opacification in early arterial phase and well delineation of a hypervascular mass in segment
5 showing intense enhancement in late arterial phase
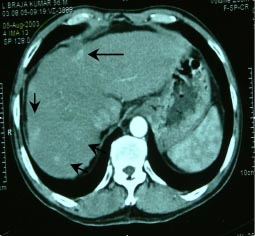
Fig. 3: Follow up MDCT, at 6 months, of a patient of
solitary HCC segment 4, treated by radiofrequency
ablation, showing recurrence along the inferomedial
margin of the defect (long arrow) with multiple fresh
enhancing lesions in segment 8 and 7 of the liver
(short arrows) seen in the late arterial phase
In addition, MDCT is also useful in the evaluation of cholangiocarcinoma (showing delayed enhancement), metastatic disease, vascular diseases like Budd-Chiari syndrome, portal vein thrombosis (better seen during portal phase), perfusion defects and vascular malformations.34,35 On account of faster subsecond scanning and the use of higher iodine concentration (370 mg/ml) for better contrast enhancement the chances of detecting hepatic lesions are significantly increased.(36) The main limitations of MDCT are large data sets and radiation concerns. The radiation dose can be reduced by the use of filters, collimation of the beam and automatic tube current modulation.(37)
In conclusion, over the years MDCT has become the imaging modality of choice for evaluating liver disease as it offers the advantage of subsecond rotation allowing faster scanning, longer scan ranges and thin collimation and better 3D depiction.
MAGNETIC RESONANCE IMAGING
MR imaging can provide comprehensive and highly accurate diagnostic information of focal liver lesions, diffuse parenchymal or depositional diseases, vascular and biliary anatomy/abnormalities, eventually obviating the need for other imaging modalities. However, movement of the diaphragm and liver during respiration is a major limitation and requires respiratory gating. This limitation has been overcome with the development of faster sequences allowing scanning in a single breath hold. Additionally, the use of 3D techniques allows multiplanar reconstructions and hence provides excellent angiographic images.
MRI provides comprehensive evaluation of liver lesions. Even though all the currently available imaging techniques cannot correctly determine the exact tumour burden in cirrhotic patients, MRI shows better sensitivity and specificity for HCC when compared with CT.(38) Administration of contrast is required in three main situations which are incidental detection of focal lesions, staging for hepatic metastasis and staging HCC in patients of cirrhosis or chronic hepatitis(39) and for early diagnosis of premalignant lesions in patients awaiting liver transplantation.(38)
Also, liver MRI when performed within six weeks following MDCT, has been found to be highly accurate for characterising subcentimetre focal liver lesions detected on MDCT.(40) Additionally, liver MRI is useful in follow up of patients of HCC treated with radioembolisation. A functional contrast-enhanced and diffusion-weighted MRI can be useful in the early post-treatment period for assessing treatment response.(41,42)
For better detection and characterisation with MRI, it is necessary to use contrast media that are capable of modifying the signal intensity of either the lesion or of the normal liver parenchyma (Figure 4).(43) The contrast agents used are of two types: the extracellular agents which are gadolinium chelates and the liver specific agents which can be hepatocyte specific (MnDPDP, Gad-EOB-BOPTA, Gad-EOB-DTPA) or Kupffer cell specific (iron containing compounds). Gadolinium is used for lesion detection and characterisation while liver specific agents are used as functional agents (Figure 4).
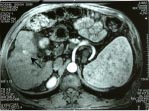
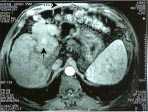
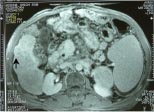
Fig. 4a,b,c: Two small masses in segment 5 of the liver on multiphasic MDCT (not shown here)
detected on routine screening in a 53-year-old cirrhotic; when the patient was subjected to
post gadolinium dynamic MRI multiple additional lesions were seen, two in segment 5
showing intense enhancement in arterial phase (a and b arrows), and another hypointense
lesion with capsular enhancement in the delayed phase (c-arrow) in segment 6 .
So far, the most commonly used substance in contrast-enhanced dynamic MR is extracellular gadolinium-chelate complex, which provides the greatest diagnostic sensitivity and specificity rates among cross-sectional techniques currently in use. Gadolinium chelates, such as Gd-DTPA, pick up lesions on account of their enhancement patterns compared with the surrounding liver parenchyma. Multiphasic post contrast MRI is similar to that of triple phase CT but has the advantage of lesser risk due to gadolinium compared with that of the iodinated contrast.
Since the vasculature is often not specific to the histological origin of focal lesions, the diagnostic accuracy of dynamic imaging alone can be unsatisfactory. Therefore, administration of contrast agents with liver-specific properties or with affinity to the reticuloendothelial system has significantly increased the accuracy of MRI for the identification and characterisation of focal liver lesions.(44,45) Also, MRI scores over CT in diagnosing the number of lesions especially in the background of chronic liver disease, a situation which poses a great diagnostic challenge for the radiologist (Figure 4).(38)
The use of superparamagnetic iron oxide (SPIO) particles is based on their distribution in the reticuloendothelial system (RES). The SPIOs locally augment the externally applied magnetic field, producing magnetic field heterogeneity, which in turn promotes dephasing resulting in signal loss from enhanced T2 relaxation. These particles are cleared from the blood via phagocytosis by the Kupffer cells of the RES, which are usually lacking in most malignant liver lesions. After SPIO injection, the signal loss of normal hepatic parenchyma surrounding focal lesions increases the contrast-to-noise ratio and depicts the lesions as markedly hyperintense on postcontrast T2-weighted images. The combined approach of non-enhanced and SPIO-enhanced T2-weighted MR images results in significantly higher sensitivity and specificity as compared to spiral CT, nonenhanced T2-weighted MR images, or SPIO-enhanced T2-weighted images alone.(46) The percentage of signal loss in focal lesions after SPIO administration can also be accurately used to differentiate benign from malignant lesions.(47)
Exclusive distribution to the hepatocellular compartment can be obtained using agents that accumulate within the hepatocytes. These properties may yield sensitivity and specificity rates of 91% and 67%, respectively, in differentiating malignant from benign lesions and rates of 91 % and 85%, respectively, in distinguishing hepatocellular from nonhepatocellular tissue.(48) But these liver specific agents do not provide information of dynamic contrast enhancement provided by gadolinium. Thus, sequential imaging in the form of gadolinium dynamic enhancement followed by liver specific agent administration can be used for better lesion detection.(27) In particular, lesions demonstrating atypical enhancement on Gadolinium-dynamic MR imaging may benefit from these twofold agent-related properties. Additionally, use of such double contrast-enhanced MR imaging helps in the depiction of advanced fibrosis in patients with cirrhosis yielding sensitivity, specificity and accuracy values greater than 90%.(49) Certain contrast agents (Gd-BOPTA & Gd-EOB-DTPA) demonstrate both extracellular and hepatocellular specific properties and thus can be used alone, instead of double-contrast, to improve detection rates of hepatocellular carcinoma, haemangioma, and metastases compared with Gd-MR imaging or dynamic CT.(50)
Thus, accurate determination of the nature of the focal liver lesions is possible using MRI with faster sequences and contrast administration.51 Combined MDCT and SPIO-enhanced MRI also has increased accuracy in pre-therapeutic evaluation of patients with HCC larger than 10mm.Combined MDCT and SPIO-enhanced MRI also has increased accuracy in pre-therapeutic evaluation of patients with HCC larger than 10mm.(52)
POSITRON EMISSION TOMOGRAPHY
Positron emission tomography (PET) is a functional imaging that entails the use of positron emitters like fluorine-18 to visualise tissues with increased glucose metabolism. The most commonly used radiotracer is 18-fluorodeoxyglucose (FDG). It is taken up by the malignant cells and remains trapped within resulting in better conspicuity of lesions, whereas, the normal cells rapidly clear FDG. FDG-PET has high tumour-to-background contrast in most malignancies with relatively high spatial resolution, which accounts for the reported high diagnostic accuracy of FDG imaging. The fusion of PET with CT images has opened up a new domain to advances in imaging.
Presently, many clinical applications of FDG- PET imaging exist. Studies have shown a high sensitivity and specificity of FDG-PET in identifying hepatic metastases from various primaries, such as colon, pancreas, oesophagus, and parotid.(53) PET and MRI have similar sensitivity and specificity in diagnosing metastasis while PET is considered the most sensitive non-invasive imaging modality for detecting liver secondaries from the gastrointestinal tract.(54,55) PET is increasingly used in pre-operative staging of malignant tumours as it plays an important role in ascertaining the extent of disease; whether localised to the liver or beyond (Figure 5).(56) Still better results can be obtained with PET-CT fusion.Still better results can be obtained with PET-CT fusion.(57)
The role of PET in the detection of HCC is less pronounced on account of the low sensitivity (50%).(58) Poorly differentiated tumours show higher uptake and are more easily detected than well differentiated ones. However use of 11C-acetate tracer allows better detection of both poorly differentiated and well differentiated HCC.(59) It is also helpful in the assessment of extrahepatic metastasis from HCC. Additionally, PET is also useful in the diagnosis of cholangiocarcinoma, in differentiation of benign from malignant gall bladder lesions and monitoring the effect of systemic therapy and in detecting residual disease after local ablative therapy for malignant focal lesions of the liver.(60) Although, PET is affected by variable FDG uptake in hepatocellular malignancies, it appears to have prognostic value depending on the standardised uptake value (SUV). For benign liver lesions like the haemangioma, the adenoma and the FNH, FDG-PET is not suitable.
In the near future, detection of subcentimetre lesions will improve with the use of PET-CT, use of small surface area crystal elements, sophisticated image correction and reconstruction algorithms, and higher spatial resolution.
Bohm et al(61) compared PET, USG, CT and MRI in the detection of focal liver lesions. They showed that contrast enhanced MRI has the best sensitivity and specificity (83% and 57% respectively). However the positive predictive value was similar for all these modalities (96-97%). For liver metastasis, MDCT and SPIO-enhanced MRI are more sensitive but less specific than PET; but PET-CT has higher sensitivity in detecting extrahepatic primary tumour.(62)
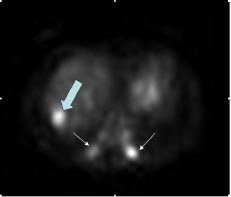
Fig.5: Increased FDG uptake in liver (large arrow)
and posterior ends of lower ribs (small arrows)
suggesting metastases seen in a 25-year-man
with known soft tissue tumour of the right shoulde
To conclude, no consensus with regard to the diagnostic algorithm for hepatic imaging exists currently. However, a variety of state-of-the-art imaging modalities like ultrasonography, CT and MRI are employed for the evaluation of hepatic abnormalities either alone or in combination depending on the patient’s history, clinical suspicion, as well as the availability of imaging modalities and the clinician’s experience of certain imaging methods. However, MRI has higher sensitivity and specificity than ultrasonography, CT and PET although CEUS and PET-CT have provided new insight in to liver imaging in recent times. Nevertheless, it is imperative to use specific contrast agents and tailored dynamic imaging protocols for obtaining optimal results.
ACKNOWLEDGEMENT
The authors would like to acknowledge Dr. Rakesh Kumar, Associate Professor, Department of Nuclear medicine, All India Institute of Medical Sciences, New Delhi, for providing the PET image.
REFERENCES
1. Daniele B, Bencivenga A, Megna AS, Tinessa V. Alfa-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–12.
2. Cosgrove D, Blomley M. Liver tumors: evaluation with contrastenhanced ultrasound. Abdom Imaging. 2004;29:446–54.
3. Blomley MJK, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001;322:1222–5.
4. Badea R, Socaciu M, Lupsor M, Mosteanu O, Pop T. Evaluating the liver tumors using three-dimensional ultrasonography. A pictorial essay. J Gastrointestin Liver Dis. 2007;16:85–92.
5. Kono Y, Mattrey R. Ultrasonography of the liver. Radiol Clin North Am. 2005;43:815–26.
6. Hohmann J, Albrecht T, Hoffmann C, Wolf K. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147–59.
7. Burns PN. Instrumentation for contrast echocardiography. Echocardiography. 2002;19:241–58.
8. Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU508A: Multicenter study. Radiology. 2003;227:361–70.
9. Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, et al. Non invasive diagnosis of hepatic cirrhosis by transit time analysis of ultrasound contrast agent. Lancet. 1999;353:1579–83.
10. Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, et al. Liver microbubble transit time compared with histology and score in diffuse liver disease: a cross sectional study. Gut. 2003;52:1188–93.
11. Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004; 232:799–809.
12. Meuwly JY, Schnyder P, Gudinchet F, Denys AL. Pulse-inversion harmonic imaging improves lesion conspicuity during US-guided biopsy. J Vasc Interv Radiol. 2003;14:335–41.
13. Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, et al. Multicentre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200– 6.
14. Wang JH, Lu SN, Changchien CS, Huang WS, Hung CH, Tung HD, et al. Flash-echo gray-scale imaging in the subtraction mode for assessing perfusion of small hepatocellular carcinoma. J Clin Ultrasound. 2003;31:451–6.
15. Wang JH, Lu SN, Tung HD, Changchien CS, Hung CH, Chen CH, et al. Flash-echo contrast sonography in the evaluation of response of small hepatocellular carcinoma to percutaneous ablation. J Clin Ultrasound. 2006;34:161–8.
16. Nicolau C, Catala V, Vilana R, Gilabert R, Bianchi L, Solé M, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–9.
17. Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, et al. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261–72.
18. Kruskal JB, Newman PA, Sammons LG, Kane RA. Optimizing Doppler and color flow US: application to hepatic sonography. Radiographics. 2004;24:657–75.
19. Liu JB, Merton DA, Goldberg BB, Rawool NM, Shi WT, Forsberg F. Contrast enhanced two and three dimensional sonography for evaluation of intra-abdominal haemorrhage. J Ultrasound Med. 2002;21:161–9.
20. Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Chung H, et al. Evaluation of post-treatment response of hepatocellular carcinoma with contrast enhanced coded phase-inversion harmonic US: comparison with dynamic CT. Radiology. 2001;221:721–30.
21. Kono Y, Lucidarme O, Choi SH, Rose SC, Hassanein TI, Alpert E, al. Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:57–65.
22. Catala V, Nicolau C, Vilana R, Pages M, Bianchi L, Sanchez M, et al. Characterization of focal liver lesions: comparative study of contrast enhanced ultrasound versus spiral computed tomography. Eur Radiol. 2007;17:1066–73.
23. Sahani D, Saini S, Pena C, Nichols S, Prasad SR, Hahn PF et al. Using multidetector CT for preoperative vascular evaluation of liver neoplasms: technique and results. AJR Am J Roentgenol. 2002;179:53–9.
24. Guiney MJ, Kruskal JB, Sosna J, Hanto DW, Goldberg SN, Raptopoulos V. Multi-Detector row CT of relevant vascular anatomy of the surgical plane in split-liver transplantation. Radiology. 2003;229:401–7.
25. Oto A, Tamm EP, Szklaruk J. Multidetector row CT of the liver. Radiol Clin North Am. 2005;43:827–48.
26. Kang HK, Jeong YY, Choi JH, Choi S, Chung TW, Seo JJ, et al. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics. 2002;22:1053–61.
27. Semelka RC, Martin DR, Balci C, Lance T. Focal liver lesions: comparison of dual-phase CT and multisequence multiplanar MR imaging including dynamic gadolinium enhancement. J Magn Reson Imaging. 2001;13:397–401.
28. Spielmann AL, Nelson RC, Lowry CR, Johnson GA, Sundaramoothy G, Sheafor DH, et al. Liver: Single breath-hold dynamic subtraction CT with multi-detector row helical technology-feasibility study. Radiology. 2002;222:278–83.
29. Nakashige A, Horiguchi J, Tamura A, Asahara T, Shimamoto F, Ito K. Quantitative measurement of hepatic portal perfusion by multidetector row CT with compensation for respiratory misregistration. Br J Radiol. 2004;77:728–34.
30. Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quiroz FA, Taylor AJ. Multiphase hepatic CT with a multirow detector CT scanner. AJR Am J Roentgenol. 2000;175:679–85.
31. Schima W, Hammerstingl R, Catalano C, Marti-Bonmati L, Rummeny EJ, Montero FT, et al. Quadruple phase MDCT of the liver in patients with suspected hepatocellular carcinoma: effect of contrast material flow rate. AJR Am J Roentgenol. 2006;186:1571–9.
32. Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US and PET. Radiology. 2003;226:533–42.
33. Kim YK, Kwak HS, Kim CS, Chung GH, Han YM, Lee JM. Hepatocellular carcinoma in patients with chronic liver disease: comparison of SPIO-enhanced MR imaging and 16-detector row CT. Radiology. 2006;238:531–41.
34. Bradbury MS, Kavanagh PV, Chen MY, Weber TM, Bechtold RE. Noninvasive assessment of portomesenteric venous thrombosis: current concepts and imaging strategies. J Comput Assist Tomogr. 2002;26:392–404.
35. Chae E, Goo H, Kim SC, Yoon CH. Congenital intrahepatic arterioportal and portosystemic venous fistulae with jejuna arteriovenous malformation depicted on multislice spiral CT. Ped Radiol. 2004;34:428–31.
36. Furuta A, Ito K, Fujita T, Koike S, Shimizu A, Matsunaga N. Hepatic enhancement in multiphasic contrast-enhanced MDCT: Comparison of high and low iodine concentration contrast medium in same patients with chronic liver disease. AJR Am J Roentgenol. 2004;183:157–62.
37. Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, et al. Strategies for CT radiation dose optimization. Radiology. 2004;230:619–28.
38. Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002; 8:749–61
39. Balci NC, Semelka RC. Contrast agents for MR imaging of the liver. Radiol Clin North Am. 2005;43:887–98.
40. Holalkere NS, Sahani DV, Blake MA, Halpern EF, Hahn PF, Mueller R. Characterization of small liver lesions: added role of MR after MDCT. J Comput Assist Tomogr. 2006;30:591–6.
41. Kamel IR, Reyes DK, Liapi E, Bluemke DA, Geschwind JF. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:49–56.
42. Deng J, Miller FH, Rhee TK, Sato KT, Mulcahy MF, Kulik LM, et al. Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolization. J Vasc Interv Radiol. 2006;17:1195–200.
43. Vogl TJ, Stupavsky A, Pegios W, Hammerstingl R, Mack M, Diebold T, et al. Hepatocellular carcinoma: evaluation with dynamic and static gadobenate dimeglumine enhanced MR imaging and histopathologic correlation. Radiology. 1997;205:721–8.
44. Kang BK, Lim JH, Kim SH, Choi D, Lim HK, Lee WJ, et al. Preoperative depiction of hepatocellular carcinoma: ferumoxidesenhanced MR imaging versus triple-phase helical CT. Radiology. 2003;226:79–85.
45. Liou J, Lee JK, Borrello JA, Brown JJ. Differentiation of hepatomas from nonhepatomatous masses: use of MnDPDP-enhanced MR images. Magn Reson Imaging. 1994;12:71–9.
46. Reimer P, Jahnke N, Fiebich M, Schima W, Deckers F, Marx C, et al. Hepatic lesion detection and characterization: value of nonenhanced MR imaging, super paramagnetic iron oxide-enhanced MR imaging, and spiral CT-ROC analysis. Radiology. 2000; 217:152–8.
47. Namkung S, Zech CJ, Helmberger T, Reiser MF, Schoenberg SO. Superparamagnetic iron oxide (SPIO)-enhanced liver MRI with ferucarbotran: efficacy for characterization of focal liver lesions. J Magn Reson Imaging. 2007;25:755–65.
48. Oudkerk M, Torres CG, Song B, König M, Grimm J, Fernandez- Cuadrado J, et al. Characterization of liver lesions with mangafodipir trisodium-enhanced MR imaging: multicenter study comparing MR and dualphase spiral CT. Radiology. 2002;223:517–24.
49. Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: Noninvasive diagnosis with double contrast material– enhanced MR Imaging. Radiology. 2006;239:425–37.
50. Petersein J, Spinazzi A, Giovagnoni A, Soyer P, Terrier F, Lencioni R, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging: a multicenter phase III clinical study. Radiology. 2000;215:727–36.
51. Semelka RC, Martin DR, Balci NC. Magnetic resonance imaging of the liver: How I do it. J Gastroenterol Hepatol. 2006;21:632–7.
52. Yukisawa S, Okugawa H, Masuya Y, Okabe S, Fukuda H, Yoshikawa M, et al. Multidetector helical CT plus superparamagnetic iron oxide-enhanced MR imaging for focal hepatic lesions in cirrhotic liver: a comparison with multi-phase CT during hepatic arteriography. Eur J Radiol. 2007;61:279–89.
53. Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK Jr, Pinson CW. Evaluation of benign vs. malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510–6.
54. Yang M, Martin DR, Karabulut N, Frick MP. Comparison of MR and PET imaging for the evaluation of liver metastases. J Magn Reson Imaging. 2003;17:343–9.
55. Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–56.
56. Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438–47.
57. Cohade C, Wahl RL. Applications of positron emission tomography/ computed tomography image fusion in clinical positron emission tomography-clinical use, interpretation methods, diagnostic improvements. Semin Nucl Med. 2003;33:228–37.
58. Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999; 94:3314–9.
59. Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–21.
60. Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–7.
61. Bohm B, Voth M, Geoghegan J, Hellfritzsch H, Petrovich A, Scheele J et al. Impact of PET on strategy in liver resection for primary and secondary liver tumors. J Cancer Res Clin Oncol. 2004;130:266–72.
62. Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, et al. Contrast-enhanced FDG PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol. 2007;48:369–78.