Deepti Shukla Misra1, Mukesh Bhardwaj1, V.P. Bhalla2, Shikha Garg1, Veena Malhotra1
Department of Histopathology1. &
Surgical Gastroentrology2.
BLK Hospital, Pusa Road,
New Delhi, India
Corresponding Author:
Dr. Deepti Shukla Misra
Email: drdeeptimisra@blkhospital.com
48uep6bbphidvals|1365 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Histiocytic sarcoma is an uncommon neoplasm with only a few reported cases till date.[1-3] Previously some lymphoid malignancies were misclassified as histiocytic lymphomas due to overlapping features and lack of immuno-histochemical evidence.[1,2,4] Here, we discuss a case report of this rare neoplasm in the terminal ileum of a female patient. The morphologic and immunohistochemical features of the neoplasm are described in detail, and its differential diagnoses excluded.
Case report
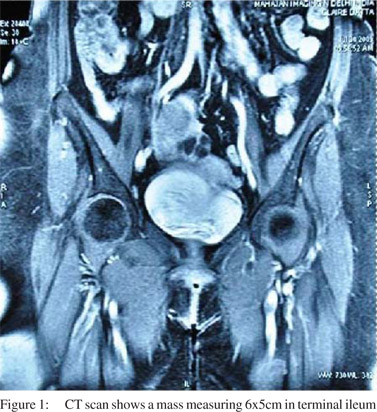
A 59-year old diabetic female presented with complaint of abdominal pain. CT abdomen showed a mass in the terminal ileum (Figure 1). PET scan was done and no other lesion was identified. Surgical resection was performed.
Result
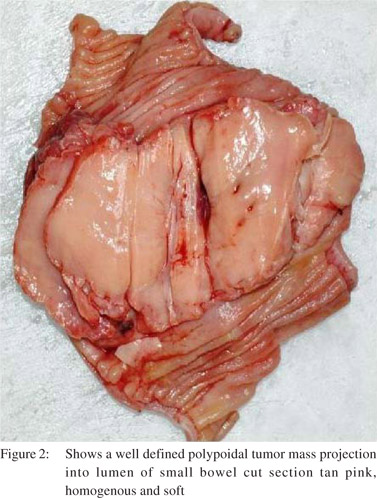
On pathologic examination, the surgical specimen consisted of a segment of small bowel. In the center of the specimen a polypoid well-defined tumor mass projecting into the lumen was seen. The tumor measured 6 cm × 5 cm. The overlying mucosa was intact. The mass extended up to the serosal surface. The cut surface was tan pink homogenous and soft. (Figure 2)
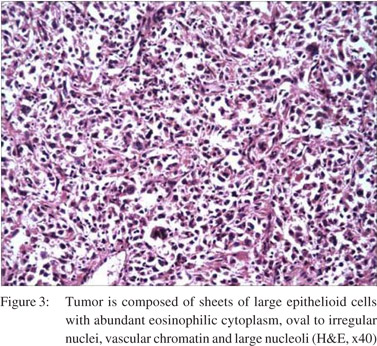
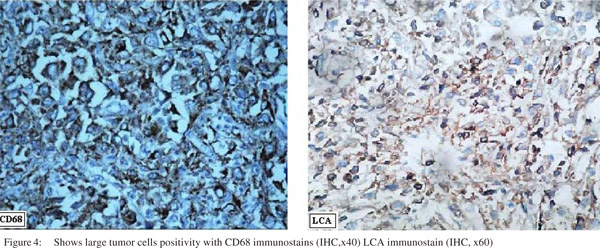
Microscopically, the tumor was composed of sheets of large epithelioid cells with abundant eosinophilic cytoplasm, oval to irregular nuclei, vesicular chromatin, and large nucleoli. Bi-nucleated cells were common. Mitoses ranged from 3-4 per 10 HPF. These cells infiltrated the muscularis propria as well as the mucosa. There was intense inflammatory infiltrate in the background. (Figure 3) On immunohistochemical examination the large tumor cells were LCA and CD68 positive. (Figure 4) The tumor cells were negative for cytokeratins, CD34, CD3, CD20, ALK-1, CD21, myeloperoxidase, HMB-45, c-kit and Desmin.
Discussion
With only a limited number of cases reported so far, Histiocytic sarcoma (HS) is a relatively new entity. Although it spans a wide age range most cases are documented in adulthood.[5] Gastro-intestinal involvement caters to only one third of the total cases.[1,2,3,5,6] It manifests in the form of abdominal mass, pain abdomen, intestinal obstruction, lower gastrointestinal bleeding or weight loss.[7] Its etiology is not known. At the present time, the World Health Organization defines HS as a malignancy with morphologic and immunophenotypic features resembling those of mature tissue histiocytes.[3,8,9] Features distinguishing malignancy from a benign histiocytic lesion include presence of malignant cytologic features with evidence of tissue invasion.[3] The latter features were seen in our case. The diagnosis of HS however relies predominantly on the verification of histiocytic lineage and the exclusion of other, poorly differentiated, large cell malignancies (i.e. lymphoma, carcinoma and melanoma) by way of extensive immunophenotypic investigation. Another strong differential includes leukemia of monocytic origin. Nonetheless, absence of a leukemic phase with circulating tumor cells supports the diagnosis of HS over the latter.
Our immunohistochemical analysis included stains to confidently exclude melanoma (i.e. melan A, HMB-45), carcinoma (i.e. cytokeratin), lymphoma (CD3, CD15, CD20, CD30, CD43, CD79a, ALK1) and accessory/dendritic cell tumors (i.e. CD1a, CD21). Since the mature tissue histiocyte is believed to be the benign counterpart of HS, CD45 (LCA) immunoreactivity is a requirement to establish hematopoietic
origin.[3] It also allowed differentiation from morphologically similar soft-tissue neoplasms. While CD45 immunoreactivity may be an absolute requirement, with the aid of additional supportive studies such as electron microscopy, differentiation from malignant fibrous histiocytoma (undifferentiated sarcoma) may be extremely difficult. These latter tumors are believed to arise from undifferentiated mesenchymal cells rather than histiocytes, and typically show pleomorphic and storiform growth patterns.[3] Despite these subtle morphologic and immunophenotypic differences, clinical history may be the only means of differentiating HS from a soft-tissue malignancy. Extranodal presentation is not that frequent and the clinical course is generally aggressive.[1-3] On the whole, unfortunately, persistent clinical and histopathologic disparities in these studies, in addition to the overall rarity of these neoplasms have continued to obscure this diagnosis and have prevented a full appreciation of their clinical behavior.
Treatment options for HS include surgery, chemotherapy and radiotherapy. Surgical resection with wide margins is the gold standard of treatment.[8] The morphologic and immunophenotypic features of HS are relatively uniform, however, an extensive panel of immunohistochemical markers is necessary to adequately exclude more common, poorly differentiated malignancies.
References
- Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004;28:1133–44.
- Avilés-Salas A, Peña-Torres ML, Molina-Cruz A, Rivas-Vera S. Histiocytic sarcoma of the small intestine. Case report and literature review. Rev Med Chile. 2009;137:269–74.
- Vos JA, Abbondanzo SL, CL Barekman, Andriko JW, Miettinen M, Aguilera NS. Histiocytic sarcoma: a study of five cases including the histiocyte marker CD163. Mod Pathol. 2005;18:693–704.
- Yoshida C, Takeuchi M. Histiocytic sarcoma: Identification of its histiocytic origin using immunohistochemistry. Intern Med. 2008;47:165–9.
- Shen XZ, Liu F, Ni RJ, Wang BY.Primary histiocytic sarcoma of the stomach: A case report with imaging findings. World J Gastroenterol. 2013;19:422–5.
- Zhang X, Kryston JJ, Michalak WA. Histiocytic sarcoma in the small intestine: a case report with flow cytometry study and review of the literature. Pathol Res Pract. 2008;204:763–70.
- Sundersingh S, Majhi U, Seshadhri RA, Tenali GS. Multifocal histiocytic sarcoma of the gastrointestinal tract. Indian J Pathol Microbiol. 2012;55:233–5.
- Machado ES, Miranda AC, Escopelli T, Caron R. Histiocytic sarcoma. Rev Bras Hematol Hemoter. 2011;33:155–7.
- Mikami M, Sadahira Y, Suetsugu Y, Wada H, Sugihara T. Monocyte/Macrophage-specific marker CD163+ histiocytic sarcoma: case report with clinical, morphologic, immunohistochemical, and molecular genetic studies. Int J Hematol. 2004;80:365–9.
|