Ujjwal Gorsi, Pankaj Gupta, Naveen Kalra, Mandeep Kang, Rajinder Singh1, Rajesh Gupta1, Vikas Gupta1, Niranjan Khandelwal
Department of Radiodiagnosis and Imaging, General Surgery1,
Post Graduate Institute of Medical Education and Research (PGIMER),
Chandigarh, India 160012
Corresponding Author:
Dr. Ujjwal Gorsi
Email: ujjwalgorsi@gmail.com
Abstract
Objective: To evaluate the role of multidetector computed tomography (CT) and CT angiography (CTA) in post cholecystectomy complications.
Methods: A retrospective analysis of data from December 2012 to August 2014 was performed. Eight hundred sixty consecutive patients with history of cholecystectomy (laparoscopic or open) were evaluated. After exclusion of 645 patients with normal imaging, analysis for post cholecystectomy complications was performed in 215 patients. A contrast enhanced CT/ CTA was performed. Mean interval to imaging was 10 months (range 3 days to 15 months).
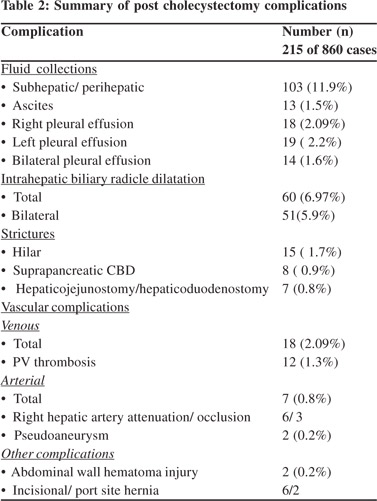
Results: A complication rate of 25% was noted in patients undergoing imaging following cholecystectomy. Gallbladder bed or perihepatic collections were seen in 11.9% cases (n=103). Intrahepatic biliary radicle dilatation (IHBRD) was seen in 7% patients (n=60). Isolated right or left ductal dilatation was seen in 9 patients; rest of the patients had bilateral IHBRD. Cholangitic abscesses and mild acute pancreatitis were seen in 11 (1.2%) and 12 (1.3%) patients respectively. These comprised biliary complications. Venous thrombosis involving the portal vein was the most frequently encountered vascular complication (n=12). Right hepatic artery pseudoaneurysm was seen in two patients. Less common complications were abdominal wall hematoma (n=2), incisional hernia (n=6), port site hernia (n=2), large bowel injury (n=1), biliocutaneous fistula (n=1) and enterocutaneous fistula (n=1).
Conclusion: CT allows classification of post cholecystectomy complications and guides further management. CTA provides an efficient road map for management of vascular complications.
|
48uep6bbphidvals|1361 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Cholecystectomy is one of the most commonly performed major surgical procedures.[1] There has been a trend shift towards laparoscopic cholecystectomy as the procedure of choice in gallstone disease.[2] Complications and their rates are somewhat different between laparoscopic and open cholecystectomy. Every step of laparoscopic cholecystectomy may potentially lead to complications.[3] Complications in open cholecystectomy are mostly related to dissection in the gallbladder bed. Biliary injury, vascular injury, retained gallstones, and abscess formation are some of the common complications in both types of cholecystectomies. Biliary complications are reported to be more common with laparoscopic cholecystectomy.[4]
Early and precise diagnosis of post cholecystectomy complications is integral to management of patients with cholecystectomies. Imaging plays a crucial role in this respect. Abdominal ultrasound (US) is often the first imaging technique employed in this setting.[5] Invariably US findings need to be supplemented with additional imaging techniques.[6] CT is a robust imaging modality as it provides good evaluation of suspected biliary and vascular injuries.[6] However, evaluation of biliary injuries benefit from additional techniques including magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC) or hepatic scintigraphy.[7] CTA provides an accurate evaluation of vascular anatomy and complications.[8] Previous studies have described the role of imaging including CT and MRI in management of post cholecytectomy complications.[3,5-8] However, advances and refinement in both the surgical technique and MDCT technology prompted us to re-explore the issue of evaluation of post-cholecystectomy complications using MDCT as the sole imaging modality.
The purpose of this study was to evaluate the role of MDCT including CTA in the evaluation of post cholecystectomy complications
Material and methods
We conducted a retrospective study to evaluate post cholecystectomy (laparoscopic as well as open) complications on abdominal contrast enhanced computed tomography (CECT). The study was approved by the local ethics committee. A total of 860 patients who were referred to our department with a history of cholecystectomy were included. CT is not routinely performed in all patients following cholecystectomy. The indication of imaging was the persistent post-operative abdominal complaints (pain, distension and constipation), fever, jaundice or suspected vascular injury. Those with equivocal findings on US also underwent CT. These patients underwent CECT abdomen from December 2012 to August 2014. The clinical data and imaging files of these patients were retrieved from our database. There were 645 patients with normal CT scans. A total of 215 patients with abnormal CT scans finally entered the analysis.
Imaging Technique
Imaging was performed on three multidetector CT scanners (Somatom Sensation 16; Somatom Definition Flash; Philips iCT). CTA was performed in patients with suspected vascular injury. CECT was performed with 100 mL of non-ionic intravenous contrast (OMNIPAQUETM 300, Iohexol, GE Healthcare, Princeton, U.S.A.) injected via 18 G cannula in the cubital fossa of upper limb. The intravenous injection was achieved with a pressure injector at a rate of 2.5 mL/s. Imaging was performed in the portal venous phase at 65 seconds from the start of contrast injection and included the area from domes of diaphragm to pubic symphysis. CTA was performed with 100 mL of omnipaque injected with a pressure injector at a rate of 4.5 mL/s. Images were acquired using bolus tracking, with the region of interest (ROI) placed in the upper abdominal aorta, and a trigger of 100 HU. The coverage for the arterial phase included T12-L3 vertebral body levels. The parameters for the three CT scanners are given in Table 1. Analysis of images was done on workstations (Syngo®.via, Siemens; Brilliance TM Workspace V2, Philips). Axial and multiple planar reformatted (MPR) images were reviewed by two radiologists with 3 and 7 years’ experience in abdominal imaging. Maximum intensity projections (MIPs) and volume rendered (VR) images were reviewed for suspected vascular injury.
Analysis of CT images
The following complications were evaluated: 1) fluid collections/ hematoma [classified based on their location into perihepatic, GB fossa (or subhepatic), abdominal wall, intraperitoneal (at other sites) or peritonitis]; 2) liver abscesses; 3) intrahepatic biliary radicle dilatation (IHBRD) (further classified as bilateral or right or left), since IHBRD can be a normal post-operative finding, it was included as a complication only when there was a biochemical correlate (raised bilirubin, alkaline phosphatase); 4) dilatation of common bile duct (CBD) with level of obstruction and cause of obstruction (classified into stricture or calculus). Similar to IHBRD, CBD dilatation was considered pathological only in the light of biochemical correlation, else it was excluded; 5) vascular complications (classified into arterial and venous, arterial complication were further segregated into pseudoaneurysm and arterial narrowing/ thrombosis); 6) suspected malignancy 7) incisional hernia or port site hernia 8) suspected bowel injury.
A fluid collection was defined as a well-defined hypodense (HU 10-45) collection while a hematoma was defined as a welldefined collection with HU more than 50. Peritonitis was present when there was ascites with abnormal enhancement of the peritoneum. Liver abscess was defined as an intraparenchymal collection showing thick enhancing wall and relatively shaggy margins. IHBRD was present when the intrahepatic biliary radicles measured more than 3 mm in calibre. CBD dilatation was defined as CBD calibre more than 10 mm (irrespective of the age of patient). The level of obstruction was classified into suprapancreatic or intrapancreatic. A stricture was defined by smooth tapering of the CBD (examined on axial and coronal reformatted images) without any obvious hyperdensity or intraluminal contents. A calculus on the other hand was identified as a rather abrupt end of the dilatation with hyperdense intraluminal contents, HU 30-60). A pseudoaneurysm was identified by a globular configuration with attenuation similar to adjacent normal arterial structure in arterial as well as venous phase. Arterial narrowing was defined by an abrupt change in calibre of the arterial structure. Similarly, occlusion was considered present when an arterial structure could not be followed beyond a certain point. Incisional hernia and port site hernia were respectively defined by herniation of
omental fat or bowel loops through the i cision (open cholecystectomy) and access port (laparoscopic cholecystectomy). Bowel injury was suspected on the basis of abnormal focal thickening of a bowel loop, extraluminal air in contiguity to an abnormally thickened loop or identification of extraluminal orally administered contrast. Clinical evaluation: Clinical history included type of cholecystectomy (open vs. laparoscopic), interval between cholecystectomy and referral for CT examination, history of any other intervention prior to imaging [viz. pigtail drainage of collections, ERCP with or without stenting, surgical history (hepaticojejunostomy/ hepaticoduodenostomy)] and availability of other imaging data .
Results
A total of 215 CT studies (including 160 CECT and 55 CTA) were evaluated. A majority of the abnormal studies were acquired on Somatom Definition Flash; Philips iCT (n=190). There were 173 females and 62 males. The mean age was 48.7 years (range 12-80). Mean interval to imaging was 10 months (range 3 days to 15 months). However in the majority of patients (n=180), imaging was performed in the first 3 months following cholecystectomy. A total of 190 laparoscopic and 25 open cholecystectomies were performed. The indication of cholecystectomy was gallstone disease (n=204), empyema (n=4), chronic cholecystitis (n=4), xanthogranulomatous cholecystitis(n=2) and GB perforation (n=1). Pigtail drainage for collections/ liver abscesses was performed in 32 patients. History of ERCP with or without stenting was present in 27 patients. Hepaticojejunostomy/hepaticoduodenostomy were performed in 7 patients for anastomotic stricture. A majority of the patients (n=165) had US examination prior to (n=130) or immediately following abdominal CT (n=35). Magnetic resonance imaging (MRI) was performed in 48 patients prior to and 30 patients following referral for CT for suspected biliary injury/ obstruction. However, neither US nor MRI findings were evaluated in the current study. This was due to the lack of uniform interval, equipment and operators for these examinations.
We analysed the complications as biliary, vascular and other.
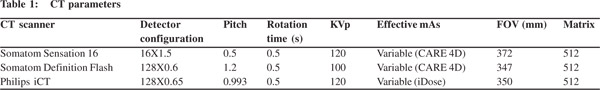
Biliary complications were divided into biliary leaks and biliary strictures. Biliary leaks were suspected on the basis of fluid collections. In total, we found 103 fluid collections. A majority of these were located in the gallbladder bed or the subhepatic location (Figure 1). The mean size of the gallbladder bed collection was 5 cm (range 2.5-12 cm). The next most common site for collections was perihepatic location (n=31). The collections in the perihepatic location were larger with mean size of 8.7 cm (range 2-20 cm). Of the 103 collections, 58 were found on CT performed in the first week following surgery.
Of these, 35 collections were less than 5 cm and were expected to resolve spontaneously. Follow up US confirmed the resolution of collections in 28 patients. In 7 patients, no follow up imaging was available. In 45 patients with persistent collections (mean CT interval 4 weeks), bile leak was suspected. In 21 patients, the diagnosis of bile leaks was confirmed based on other imaging modalities (viz. MRCP, ERCP, nuclear scans) and was managed by stenting (n=17) and surgery (n=4). We encountered one case of biliary peritonitis.
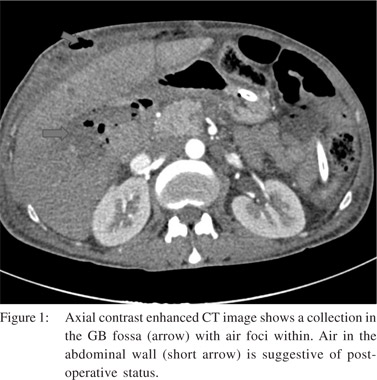
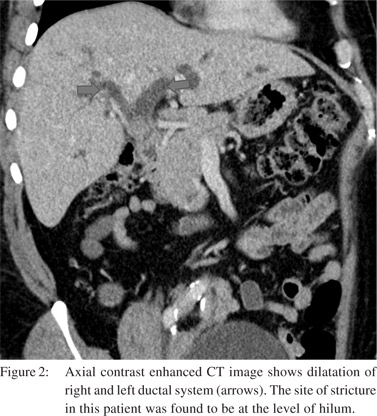
We found IHBRD in 60 patients. Of these the majority had bilateral IHBRD (n=51) and the underlying cause was a hilar stricture in 15 (Figure 2), suprapancreatic CBD stricture in 8 and stricture at hepaticojejunostomy (n=6) and hepaticoduodenostomy sites (n=1). CBD calculi were detected in 17 patients. Biliary strictures were a relatively common complication. In 9 patients, there was isolated right (n=6) or left IHBRD (n=3) due to strictures involving secondary confluence. Mean interval to imaging in patients presenting with biliary strictures was 7 months (mean 2.5-14 months). Cholangitic abscesses secondary to biliary stricture were found in 11 patients (Figure 3). Mild acute pancreatitis was detected in 12 patients.
Vascular complications were relatively less common. Venous thrombosis was the most common complication (n=18) in our study. PV thrombosis was seen in 12 patients (Figure 4).
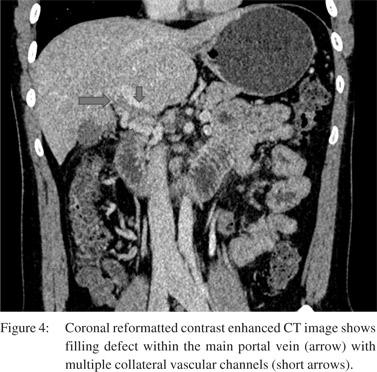
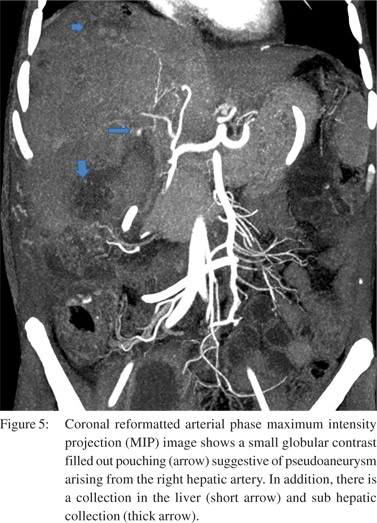
Of these, 2 patients had isolated right portal vein thrombosis. Splenic vein and SMV thrombosis were found in 3 patients and 1 patient, respectively. Arterial complications involved only the right hepatic artery. Attenuated right hepatic artery was seen in 6 patients. Three patients had occlusion of right hepatic artery. Pseudoaneurysm of the right hepatic artery was detected in two patients (Figure 5). Mean interval to imaging in patients with venous thrombosis was 3 months (mean 1 to 10 months).
Other complications included abdominal wall hematoma (n=2), incisional hernia (n=6), port site hernia (n=2), large bowel injury (n=1), biliocutaneous fistula (n=1) and enterocutaneous fistula (n=1). Right pleural effusion was seen in 18 and left pleural effusion in 19 patients. Bilateral effusion was seen in 14 patients. Ascites was present in 13. Nine patients had both pleural effusion and ascites. The various complications are summarised in Table 2.
No significant difference in the biliary and vascular complications was noted between the open and laparoscopic cholecystectomy group. Complications that were noted in the laparoscoic cholecystectomy group included port site hernia and abdominal wall hematoma.
Discussion
Laparoscopic cholecystectomy is the procedure of choice for gall stone disease.[2] The advantages over open cholecystectomy include a shorter hospital stay and shortened recovery periods.[9] Even in the present era when laparoscopic cholecystectomy has become the gold standard, open cholecystectomy is still performed either as a primary procedure or as a conversion procedure. The latter is more common and indications of conversion of laparoscopic to open cholecystectomy include inflammation, adhesions and difficult anatomy.[10] Complications and their rates differ between the two types of operations. Complications are associated with virtually every step of the procedure in laparoscopic cholecystectomy.[3] Common complications in both types of procedures include biliary injury, vascular injury, retained gallstones, and abscess formation.[11] Biliary complications are reportedly more common inlaparoscopic cholecystectomy.[4] This is particularly true for the less experienced surgeon.[12] However, complication rates are comparable for experienced operators. This difference was not statistically significant in the present study. This could be attributed to an overall lower rate of open cholecystectomy and local expertise with both the techniques.
Early diagnosis must be made to reduce morbidity and prolonged hospital stay. Imaging plays an important role. US, CT, MRCP, ERCP, PTC and hepatobiliary scintigraphy are the potential imaging modalities employed in these patients. Ultrasonography is the initial investigation performed in patients suspected of developing post cholecystectomy complications.[5] MRCP, ERCP and PTC are mainly used for biliary complications.[7] ERCP and PTC are invasive and employed when MRCP is contraindicated or intervention is contemplated. Hepatobiliary scintigraphy is used less frequently. Its main role is to diagnose and localise bile leak.[13,14] Although not effective as an independent modality, particularly in evaluation of patients with biliary complications, CT provides a good guide to further imaging. CT, however, is quite effective in diagnosing vascular complications, abscesses and other rarer complications. It has the advantage of easy availability and shorter procedure time. Previous studies have described the role of imaging including CT and MRI in managing postcholecytectomy complications. [3,5-8,16] However, advances and refinement in both the surgical technique and MDCT technology prompted us to re-explore the issue of evaluation of post-cholecystectomy complications using MDCT as the sole imaging modality.
Bile duct injury often goes unrecognized at the time of surgery.[15,16] The rate of biliary injury ranges from 0.2% to 7% in laparoscopic compared to 0.2-0.4% following open cholecystectomy.[4] Acute injuries manifest as leaks, whilst chronic injuries lead to strictures. In the acute phase, CT shows perihepatic fluid collections or free intraperitoneal fluid. However, fluid collections in the surgical bed are common, occurring in up to 14% of patients.[17] These tend to resolve spontaneously. We found a comparable incidence of such collections (16%). Biliary leak should be suspected when a collection persists for more than 1 week or occurs outside the gallbladder bed.[18] Kirks et al evaluated the role of CT cholangiography in the detection and localisation of suspected bile leaks. Over a period of 9 years, the authors evaluated 20 patients with suspected bile leaks. Seven patients underwent CT cholangiography as the primary imaging modality. A preoperative diagnosis of bile leaks based on CT cholangiography was established in 6 patients and excluded bile leak in 1. In the present study, no CT cholangiography studies were performed. Following cholecystectomy, strictures result from misplaced clips or thermal injury during cautery. Strictures are seen on CT as IHBRD with focal smooth narrowing of the bile duct. The most common pattern is dilatation of ducts in both lobes; however, isolated dilatation of ducts in a single lobe is also possible.[19] We also found a similar trend with bilateral IHBRD in 23% patients and isolated right and left IHBRD in 2.7% and 1.8% patients respectively. However, if there is no biliary dilatation, these may go unrecognised. Several studies have evaluated the role of CT in demonstration of biliary strictures.
Most of these studies have included the post orthotopic liver transplant (OLT) patients. Zoefp et al evaluated 75 patients suspected with biliary injury following OLT using US, CT, MRI/ MRCP and ERCP. They reported a sensitivity and specificity of 40% and 57.1% (compared to 71% and 25% for MRI) for anastomotic site stricture and sensitivity of 83.3% (compared to 88.9% for MRI) for ischemic type biliary lesions.[20] Rosch et al compared the diagnostic accuracy of ERCP, MRCP, CT and EUS in biliary strictures. Fifty patients, including 17 with benign strictures, were studied. A sensitivity and specificity of 85%/ 75% for ERCP/PTC, 85%/71% for MRCP, 77%/63% for CT, and 79%/62% for EUS was reported for prospective diagnosis of malignancy.[21] Muraoka et al reported three cases where thinslice CT was utilised for demonstration of biliary stricture following abdominal trauma.[22] The suprapancreatic portion of the common bile duct was detected in all cases. Zeman et al demonstrated the role of 3-D helical CT in the evaluation of the site and length of stricture in 6 patients. The results were compared with those of ERCP and PTC. They found a good correlation between these modalities and CT. They concluded that 3-D CT is useful in surgical planning.[23] Based on the results of ERCP, we found a sensitivity and specificity of 85%/65% for detecting of biliary strictures. Besides strictures, biliary dilatation can also result from retained gallstones, papillary stenosis or biliary dyskinesia.[24] Stones are visible on CT only if they have a differential density compared to bile.[25] Kim et al conducted a study to assess the pattern and detectability of biliary stones on CT. In a study of 191 patients, CT showed a sensitivity of 85.4% compared to ERCP. They concluded that the detectability of CBD stone depends on the type and size of stone. The size cut-off was 5mm.[26] Thus, contrary to popular thinking, CT shows moderate sensitivity and specificity in detecting biliary strictures and stones. No large studies, however, are available in the post-cholecystectomy setting. We found CBD stones as a cause of CBD dilatation in 8% patients. However, our results are limited by lack of ERCP confirmation in all cases. It can be anticipated that despite being inferior to MRCP and ERCP in its diagnostic performance, CT answers several issues unlike the former two and is easily applicable in emergency setting. Thus it can act as a one-stop shop in evaluation of post-cholecystectomy complications. It may guide further appropriate evaluation. Vascular complications during laparoscopic cholecystectomy most frequently involve the gallbladder fossa or the abdominal wall.[27] These complications are secondary to trocar insertion.[28] During trocar insertion, small abdominal wall vessels (most commonly inferior epigastric vessels) may be injured, leading to abdominal wall hematoma. Less commonly mesenteric vessels may be injured producing a mesenteric hematoma. Rare sites of vascular injury are inferior vena cava and abdominal aorta.[29] Abdominal wall or intra-abdominal hematomas appear as hyperdense collections on CT.[30] The size of these hematomas ranges from small and clinically insignificant to large and potentially life-threatening. Dissection in the gallbladder bed causes injury to local vessels, most commonly, right hepatic artery and portal vein. Hepatic artery injury takes the form of pseudoaneurysm formation, attenuation or occlusion. Portal vein injury most commonly causes attenuation or thrombosis.[31] Arterio-portal fistula is a rare complication. Alves et al studied 55 patients following cholecystectomy for vascular injuries.[32] Disruption of the right hepatic artery or of a replaced right hepatic artery was detected in 20 patients (36%), pseudoaneurysm of the right branch of the hepatic artery in 2 (4%), and portal vein injury with (n = 3) or without (n = 1) hepatic artery injury in 4 patients (7%). This was a catheter angiographic study and the study group comprised of patients referred for biliovascular injury. The role of CT angiography in detecting vascular complications has been described in case reports only.[33] With significant advances in CT technology, it is expected to yield a sensitivity and specificity similar to catheter angiography. We found right hepatic artery attenuation/occlusion in 4% and right hepatic artery pseudoaneurysm in 1% patients. Main portal vein thrombosis was seen in 5.5% cases. CT angiography is the initial modality of choice as it is non-invasive and provides exquisite details of the vascular anatomy.8 This provides a road map for catheter angiography performed for endovascular therapeutic procedures or surgical management.
Few complications are specific to laparoscopic cholecystectomy which include :.port site hernia, diaphragmatic hernia, splenic rupture, intestinal ischemia or perforation. Port site hernia is a rare complication and is seen as herniation of omental fat or small bowel. It has a variable incidence ranging from 0.02 to 0.36%.[34] Diaphragmatic hernia results from inadvertent injury to the diaphragm during trocar insertion.[35] The reported incidence of bowel injury is between 0.14 and 0.35%.[36] A similar incidence for these rare complications was found in our study. Additionally, we report one case each of biliocutaneous and enterocutaneous fistulas.
In conclusion, CT is a robust imaging tool in evaluation of patients following cholecystectomy. It allows precise detection of virtually all possible complications with the advantage of easy availability and shorter imaging time.
References
- Litwin DE, Cahan MA. Laparoscopic cholecystectomy. Surg Clin North Am. 2008;88:1295–313.
- Wu JS, Dunnegan DL, Luttmann DR, Soper NJ. The evolution and maturation of laparoscopic cholecystectomy in an academic practice. J Am Coll Surg. 1998;186:554–61.
- Ray CE Jr, Hibbeln JF, Wilbur AC. Complications after laparoscopic cholecystectomy: imaging findings. AJR Am J Roentgenol. 1993;160:1029–32.
- Kaman L, Sanyal S, Behera A, Singh R, Katariya RN. Comparison of major bile duct injuries following laparoscopic cholecystectomy and open cholecystectomy. ANZ J Surg.2006;76:788–91.
- McGahan JP, Stein M. Complications of laparoscopic cholecystectomy: imaging and intervention. AJR Am J Roentgenol. 1995;165:1089–97.
- Slanetz PJ, Boland GW, Mueller PR. Imaging and interventional radiology in laparoscopic injuries to the gallbladder and biliary system. Radiology. 1996;201:595–603.
- Lohan D, Walsh S, McLoughlin R, Murphy J, et al. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol.2005;15:904–12.
- Kim JY, Kim KW, Ahn C, Hwang S, Lee Y, Shin YM, et al. Spectrum of biliary and nonbiliary complications after laparoscopic cholecystectomy: Radiologic findings. AJR Am J Roentgenol. 2008;191:783–9.
- Gadacz TR, Talamini MA. Traditional versus laparoscopic cholecystectomy. Am J Surg. 1991;161:336–8.
- Ishizaki Y, Miwa K, Yoshimoto J, Sugo H, Kawasaki S. Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br J Surg. 2006;93:987–91.
- Fathy O, Zeid MA, Abdallah T, Fouad A, Eleinien AA, el-Hak NG,et al. Laparoscopic cholecystectomy: a report on 2000 cases. Hepatogastroenterology. 2003;50:967–71
- Hobbs MS, Mai Q, Knuiman MW, Fletcher DR, Ridout SC. Surgeon experience and trends in intraoperative complications in laparoscopic cholecystectomy. Br J Surg.2006;93:844–53.
- Shikare SV, Supe AN, Tilve GH. Scintigraphic detection of bile leak and followup in a postcholecystectomy patient with recognition of tail sign. J Postgrad Med. 1995;41:15–7.
- Tripathi M, Chandrashekar N, Kumar R, Thomas EJ, Agarwal S, Bal CS,et al. Hepatobiliary scintigraphy: an effective tool in the management of bile leak following laparoscopic cholecystectomy. Clin Imaging.2004;28:40–3.
- Bingham J, McKie LD, McLoughlin J, Diamond T. Biliary complications associated with laparoscopic cholecystectomy: an analysis of common misconceptions. Ulster Med J.2000;69:106–11.
- Slanetz PJ, Boland GW, Mueller PR. Imaging and interventional radiology in laparoscopic injuries to the gallbladder and biliary system. Radiology.1996;201:595–603
- Schofer JM. Biliary causes of postcholecystectomy syndrome. J Emerg Med. 2010;39:406–10
- Girometti R, Brondani G, Cereser L, Como G, Del Pin M, Bazzocchi M,et al. Post-cholecystectomy syndrome: spectrum of biliary findings at magnetic resonance cholangiopancreatography. Br J Radiol. 2010;83:351–61
- BuxtonThomas M, Chisholm R, Dixon AK. Intrahepatic bile duct dilatation shown by computed tomography: predilection for the left lobe? Br J Radiol.1985;58:499–502.
- Zoepf T, Maldonado-Lopez EJ, Hilgard P, Dechêne A, Malago M, Broelsch CE, et al. Diagnosis of biliary stricture after liver transplantation: which is the best tool? World j Gastroenterol. 2005;11:2945–8.
- Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, et al. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endoscop. 2002;55;870–6.
- Muraoka N, Sakai T, Koneri K, Imamura Y, Sagoh T, Okuizumi Y, et al. Thin slice CT findings of biliary stricture due to blunt abdominal trauma: reports of three cases. Jpn J Radiol. 2013;31:500–4
- Zeman RK, Berman PM, Silverman PM,Cooper C, Garra BS, Patt RH, et al. Biliary tract: three-dimensional helical CT without cholangiographic contrast material. Radiology. 1995;196:865–7.
- Piccinni G, Angrisano A, Testini M, Bonomo GM. Diagnosing and treating shpincter of Oddi dysfunction –a critical literature review and reevaluation. J Clin Gastroenterol. 2004;38:350–9.
- Thurley PD, Dhingsa R. Laparoscopic cholecystectomy: postoperative imaging. AJR Am J Roentgenol. 2008;191:794–801.
- Kim CW, Chang JH, Lim YS, Kim TH, Lee IS, Han SW.et al. common bile duct stones on multidetector computed tomography: Attenuation patterns and detectability. World J Gastroenterol. 2013;19:1788–96.
- Shamiyeh A, Wayand W. Laparoscopic cholecystectomy: early and late complications and their treatment. Langenbecks Arch Surg. 2004;389:164–71.
- Schäffer M, Lauper M, Krähenbühl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15:275–80.
- Usal H, Sayad P, Hayek N, Hallak A, Huie F, Ferzli G. Major vascular injuries during laparoscopic cholecystectomy. Surg Endosc.1998;12:960–2.
- Geraci G, Sciumè C, Pisello F, Li Volsi F, Facella T, Modica G. Trocarrelated abdominal wall bleeding in 200 patients after laparoscopic cholecystectomy: personal experience. World J Gastroenterol.2006;12:7165–7
- Preventza OA, Habib FA, Young SC, Penney D, Oppat W, Mittal VK. Portal vein thrombosis: an unusual complication of laparoscopic cholecystectomy. JSLS. 2005;9:87–90
- Alves A, Farges O, Nicolet J, Watrin T, Sauvanet A, Belghiti J.Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct stricture. Ann Surg. 2003;238:93–6.
- Rencuzogullari A, Okoh AK, Akcam TA, Roach EC, Dalci K, Ulku A. Hemobilia as a result of right hepatic artery pseudoaneurysm rupture: An unusual complication of laparoscopic cholecystectomy. Int J Surg Case Rep. 2014;3:142–4.
- Bergemann JL, Hibbert ML, Harkins G, Narvaez J, Asato A. Omental herniation through a 3mm umbilical trocar site: unmasking a hidden umbilical hernia. J Laparoendosc Adv Surg Tech A. 2001;11:171–3.
- Armstrong PA, Miller SF, Brown GR. Diaphragmatic hernia seen as a late complication of laparoscopic cholecystectomy. Surg Endosc.1999;13:817–8.
- Shea JA, Healey MJ, Berlin JA, Clarke JR, Malet PF, Staroscik RN,et al. Mortality and complications associated with laparoscopic cholecystectomy. Ann Surg. 1996;224:609–20.
|