Olith Selvan, Mukul Vij, Gomathy Narasiman, Venkatkrishnan L1, Anand Bharathan, Mohammed Rela
Department of Histopathology and Liver Transplantation and Hepatobiliary Surgery1,
Global Hospitals and Health City,
Perumbakkam, Chennai,
India-600100
Corresponding Author:
Dr. A Olith Selvan,
Email: olith2000@yahoo.com
48uep6bbphidvals|1354 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext We present an unusual case of sarcoidosis presenting with biliary cirrhosis requiring liver transplantation.
Case report
A 52-year-old man presented with jaundice and abdominal distention in May 2011 and underwent further investigations in an outside hospital. His liver function showed a bilirubin of 2.8mg/dL, ALT 26U/L, AST59 U/L, alkaline phosphatase 236U/L, albumin 2.1gm/L, globulin 4.9gm/dL and GGT 80U/L. His CT scan abdomen showed cirrhotic liver with free fluid and splenomegaly. Investigations for chronic liver disease etiology revealed a positive AMA with a titre of 1:100. Other autoantibodies and viral serological markers were negative. He had a percutaneous needle liver biopsy which showed advanced fibrosis, expanded portal tract and inflammatory infiltrate mainly composing lymphocytes with insignificant bile duct damage. There was also the occasional periportal non-caseating epitheloid granuloma. A diagnosis of primary billiary cirrhosis was considered. He later presented with spontaneous bacterial peritonitis (SBP) and septicemia and subsequently had a lifethreatening variceal bleed, which required intubation and ventilation for management. He was transferred to our center for consideration of liver transplant. A pre-transplant work up was undertaken and the patient was placed on the waiting list for deceased donor liver transplantation (DDLT). During follow up in the waiting period, thrombus in the IVC was noted. The patient underwent liver transplantation. During the operation a shrunken, nodular and firm liver was seen, with hugely hypertrophied caudate lobe. Anorthotopic liver transplantation was done along with classical caval replacement and inferior vena caval thrombectomy.
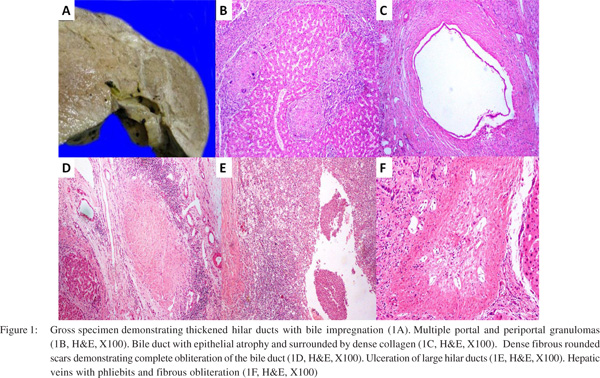
The explant liver weighed 1400g and measured 20 cm x 15 cm x 10cm. The cut surface was firm, nodular, and showed diffuse fibrosis. The hilum showed few dilated hilar ducts containing bilirubin casts (Figure 1A). The sections showed biliary cirrhosis with marked distortion of portal and lobular architecture with formation of variable sized regenerative nodules demarcated with fibrous septa. Foci of bridging fibrosis and incomplete nodularity were also noted. The portal tracts and septa were expanded and showed prominent bile ductular reaction, ectatic vascular channels, with moderate inflammatory cell infiltrate comprising of lymphocytes, few plasma cells along with some polymorphs and occasional eosinophils. Multiple lymphoid aggregates were noted. Multiple portal and periportal granulomas composed of epithelioid histiocytes admixed with multinucleated giant cells in few were noted (Figure 1B). Few granulomas show central granular necrosis. Periductal fibrosis with epithelial atrophy was also noted in multiple portal tracts (Figure 1C).Distorted septal and larger ducts were noted. Larger portal tracts showed loss of bile ducts, the site indicated by fibrous rounded scars (Figure 1D). There was ulceration of large hilar ducts with cholangitic abscesses, duct wall thickening with bile impregnation and intrahepatic bile sludge (Figure 1E).
Xanthogranulomatous cholangitis, a lesion consisting of exuberant granulation tissue with prominent foamy macrophages, acute and chronic inflammation, fibrosis and foreign-body giant cells, were also found at the liver hilum. Hyperplastic arterioles were noted. Portal vein intimal fibrosis was also noted. Few foci show spill over of portal/septal inflammation into adjacent periportal/periseptal parenchyma.
The hepatic parenchyma showed focal ballooning of hepatocytes and patchy lobular inflammation with spotty necrosis. There were sinusoidal dilatation, lymphocytosis and hyperplastic Kupffer cells. Scattered apoptotic hepatocytes were noted in nodules. Multiple lobular granulomas composed of epithelioid histiocytes admixed with multinucleated giant cells were identified. Occasional asteroid bodies were also noted in giant cells. Few hepatic veins showed phliebits with fibrous obliteration (Figure 1F). The granulomas showed increased reticulin fibres. The sections from hilar region showed cystically dilated and ulcerated/ruptured large ducts, with biliary sludge, and xanthogranulomatous inflammatory reaction along with veins and arteries. Focal squamous metaplasia of the duct epithelium was noted. The duct also showed periductular fibrosis with epithelial atrophy. There was no significant iron overload. There was increase in stainable copper/copper associated protein in periportal/periseptal hepatocytes (Grade 2/3). The final impression was released as hepatobiliary sarcoidosis with secondary sclerosing cholangitis with no morphological evidence of PBC.
Discussion
Liver disease due to sarcoidosis is probably under reported. Hepatic involvement by sarcoidosis is highly variable but the patterns of hepatic injury have been well described.[1] Three major histological patterns are described including chronic intrahepatic and rarely extrahepatic cholestasis, necroinflammatory and vascular changes.[1,2] Granulomatous involvement of the bile ducts resembles primary biliary cirrhosis. Periductal fibrosis with epithelial atrophy and nodular scars resembles primary sclerosing cholangitis. Cholestatic changes also include progressive diminution in the number of interlobular bile ducts, periportal fibrosis, and the eventual development of a micronodular biliary cirrhosis.[3]Occasional cases of cholestasis in sarcoidosis are the result of a mass effect of sarcoid nodules at the hilum of the liver with bile duct obstruction.Vascular changes in hepatic sarcoidosis manifest mainly in sinusoidal dilatation/congestion and nodular regenerative hyperplasia.[4] Granuloamatous phelibits may also be noted. The classic granuloma in sarcoidosis is mainly found in the portal triads with a cluster of large epithelioid cells, often with multinucleated giant cells. The granulomas of sarcoidosis may exhibit focal granular necrosis of minimal amount.[1] Incases with granulomas that exhibit a greater degree of necrosis an infective etiology should be strongly suspected. A variety of inclusions may be present, including Schaumann’s bodies, asteroid bodies, birefringent crystals, and Hamazaki-Wesenberg bodies. The granulomas of sarcoid are reticulin rich.
The present case also posed a diagnostic challenge between the chronic cholestatic syndrome of sarcoidosis and primary biliary cirrhosis considering the serological positivity of AMA.A positive mitochondrial antibody titer is characteristic of primary biliary cirrhosis and is present in > 90% of cases. On rare occasions, differentiation between sarcoidosis and primary biliary cirrhosis may be impossible because cases have been described with clinical and histologic features of both conditions where mitochondrial antibody was positive or negative.[1,5]AMAs are usually absent in patients with sarcoidosis and cholestatic liver disease. To be precise, however, anti-M2 AMAs are specific for PBC. Non-M2 AMAs are found in a small percentage of normal individuals. The widely used commercial immuno-fluorescence tests for AMAs do not distinguish between M2 and non-M2 auto antibodies. Instead, a titer above 1:40 is used as an arbitrary cutoff value to identify patients with PBC. Our patient had an AMA titer of 1:100, well above the cutoff value. There is an overlap of the histopathology of these two conditions, although they differ in their typical forms5. In primary biliary cirrhosis, there is non suppurative destructive cholangitis also termed as florid duct lesion. Granulomas are usually few in number, poorly defined and are usually associated with bile ducts. Comparatively in sarcoidosis, if bile duct damage is present it is less conspicuous; granulomas are abundant and well-formed. Lobular granuloma commonly occur in sarcoidosis and are rare in PBC.
References
- Devaney K, Goodman ZD, Epstein MS, Zimmerman HJ, Ishak KG. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol. 1993;17:1272–80.
- Ishak KG. Sarcoidosis of the liver and bile ducts. Mayo Clin Proc. 1998;73:467–72.
- Ilan Y, Rappaport I, Feigin R, Ben-Chetrit E. Primary sclerosing cholangitis in sarcoidosis. J Clin Gastroenterol. 1993;16:326–8.
- Kakar S, Kamath PS, Burgart LJ. Sinusoidal dilatation and congestion in liver biopsy: is it always due to venous outflow impairment? Arch Pathol Lab Med. 2004;128:901–4.
- Stanca CM, Fiel MI, Allina J, Caracta CF, Odin JA. Liver failure in an ant mitochondrial antibody-positive patient with sarcoidosis: primary biliary cirrhosis or hepatic sarcoidosis? Semin Liver Dis. 2005;25:364–70.
|