|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
Venous thromboembolism, Ulcerative colitis, Inflammatory bowel disease, Thrombosis, Deep vein thrombosis, Cerebralvenous thrombosis. |
|
|
|
Brij Sharma1, Neetu Sharma2, Vineeta Sharma3, Vishal Bodh1, Rajesh Sharma1, Rajesh Kumar1, Sidhant Sharma4, Harmandeep Singh Thabal1, Ashish Chauhan1 1Department of Gastroenterology, 2Department of Physiology, 3Department of Microbiology, Indira Gandhi Medical College, Shimla, India. 4MBBS Student, Anna Medical College, Mauritius.
Corresponding Author:
Dr. Ashish Chauhan Email: marquez.dvl@gmail.com
DOI:
http://dx.doi.org/10.7869/tg.744
Abstract
Background: Venous thromboembolism (VTE) is a known complication in patients with inflammatory bowel disease (IBD), including ulcerative colitis (UC). This study aimed to investigate the prevalence and clinical characteristics of VTE in patients with UC, and to identify potential risk factors for VTE in this population. Methods: This was a single-centre retrospective cohort study involving 167 patients with UC from 2015 and 2022. Patient demographics, disease characteristics, medication history, and VTE events were extracted from outpatient records. Results: The study included 167 patients, of which 58.68% were males. The prevalence of VTE in outpatients with UC was 3% (n=5), with four patients having deep venous thrombosis (DVT), two having cerebral venous thrombosis (CVT) and one patient having both CVT and DVT. The mean age of patients with VTE was 48 ± 18.75 years and all patients were females. Four patients had history of acute severe colitis in past and were treated with steroids. There was no mortality in patients with VTE during the study period, and none underwent colectomy. Prothrombotic work up was negative in all the patients (4/4). The median duration of follow-up was five years, and median duration between IBD diagnosis and occurrence of VTE was three years. Conclusion: This study found a prevalence of 3% in a cohort of UC patients with milder disease. Past history of acute severe colitis and steroid use were seen in patients with UC who developed VTE (4/5), though no statistically significant association could be found due to small sample size.
|
Introduction
Venous thromboembolism (VTE) comprises of interrelated disorders of deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) or rarely cerebral venous thrombosis (CVT) or thrombosis of other abdominal veins.1,2 VTE is usually precipitated by an underlying condition or an acute event with dysregulated coagulation as its pathophysiological basis.3 It is an under-recognized disorder with significant mortality.4,5 DVT and PTE are associated with a short-term mortality of 6% and 20%, respectively, with underlying disease being an important variable.6,7 Inflammatory bowel disease (IBD), both ulcerative colitis (UC) and Crohn’s disease (CD), is associated with increased risk of thromboembolic events.8 A recent metanalysis of 11 studies had shown a 2-fold increased risk of VTE in patients with IBD.9 In a large cohort of 2784 IBD patients, 90% of VTE episodes were either DVT and/or PTE, and 10% were cerebral, portal, splenic, mesenteric or jugular venous thrombosis.2 Various studies have shown differing prevalence rates of VTE in patients with IBD ranging from 1-6%4,5,10-12. In the Indian subcontinent, previous studies have reported a prevalence of VTE in IBD at 0.9%. The predictors for occurrence of VTE in IBD, in that study included pancolitis [hazard ratio (HR) = 9.7], steroid dependent/refractory disease course (HR = 3.1) and history of acute severe colitis (HR = 13.7).10 Another study of 59 ulcerative colitis patients identified pancolitis and active disease as risk factors for VTE, with 76% and 80% of VTE patients exhibiting these characteristics, respectively.11 Additionally, a large study found that the relative-risk of VTE in IBD compared to controls was four-fold higher, higher in patients during flares (HR = 8.4) and increased to 15-fold higher in ambulatory patients during flares.12 The increased propensity for VTE in IBD has been attributed alterations in the levels of procoagulant, anti-fibrinolytic, anti-coagulation and pro-fibrinolytic factors, although the exact underlying mechanism remains unclear.13 The risk of VTE is more during periods of active disease and hospitalization, with studies consistently demonstrating a higher prevalence of VTE in patients with active IBD.2,4,10,14 In addition to the traditional risk factors for venous thromboembolism (VTE) such as gender, age, recent surgery, oral contraceptives (OCPs), pregnancy and malignancy, specific risk factors associated with IBD further increase this risk [disease activity - IBD flare (HR=8.4), disease extent – pancolonic involvement in 76%, surgery – IBD-related surgery (HR = 40), systemic corticosteroids, hospitalization].2,4,10,15,16 A metanalysis involving 3110 studies reported a relative risk of 3.5 for combined OCPs versus non-users, with the risk of venous thrombosis being influenced by both the type of progesterone and the dose of estrogen.18 VTE may present with non-specific symptoms and hence, threshold for suspicion of VTE should be low in patients with IBD.19 Most of the existing data on VTE in IBD comes from inpatient discharges or large databases of tertiary care centers, which may not reflect the characteristics of patients who receive care in outpatient settings.2,4,10 Outpatients may represent a different subset who have a milder disease and may have different risk factors compared to hospitalized patients. Scoville et al., utilizing a case-control design, noted that within the subgroup of outpatients, the risk factors for VTE were recent hospitalization (within last 4 weeks), steroids usage and active disease during out patient care.17 Hence, we planned this retrospective study to characterize the risk of VTE in our patients (outpatients) of UC to know the prevalence, type of thrombosis, risk factors and its relation to the disease activity.
Methods
Study Design
This study involved a retrospective analysis of the inflammatory bowel disease (IBD) database at our centre, primarily focusing on outpatients. The database was maintained by author BS in electronic format. The study population comprised of adult patients aged 18 years or older who received follow-up care at our centre between January 2015 and April 2022.
Patients
This study included all patients who received a diagnosis of ulcerative colitis as per European Crohn’s and Colitis Organization (ECCO) guidelines and followed-up under department of gastroenterology during the study period.20 The diagnosis of VTE was obtained from patient records and likely, only patients with symptoms of vascular thrombosis were investigated for thrombosis, a limitation we acknowledge. Comprehensive patient details, including demographics, disease course, disease status, biochemical investigations, endoscopic findings and therapy details were retrieved from the records. There was incomplete data regarding certain variables such as the use of OCP or steroids in past, past history of acute severe colitis, body mass index and medications used for UC. So, data regarding these variables were not included for baseline characteristics. For patients with VTE (n=5), data on these variables were collected again via telephone. History of corticosteroid prescription in past was taken as a surrogate for disease flare. Simple Clinical Colitis Activity Index (SCCAI) of = 2, 3-6, 7-9, >10 was used to classify disease activity as remission, mild, moderate and severe.21 Steroid dependent/refractory disease was defined as per ECCO guidelines.22 The ethical clearance for the study was obtained from institute ethics committee via noHFW (MC-II)B (12) ETHICS/2020/-16039. The STROBE guidelines were followed for reporting observational studies.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) depending upon normal or non-normal distribution. Categorical variables were expressed as percentages. P-value < 0.05 was considered statistically significant. Stata software (version 14) was used for analysis.
Results
Baseline Characteristics
A total of 167 patients were included in the study, of which 58.68% (98/167) were males. The median age of study population was 46 (IQR: 32-58) years. The baseline characteristics of study population are provided in Table 1. The extent of disease as proctitis, left sided colitis and pancolitis was seen in 28.05%, 32.32% and 39.69% of patients, respectively. Diabetes and hypertension were seen in 18.56% and 28.74% of patients, respectively. However, data regarding smoking and OCP use was not available in the records. About 50% patients had either biochemical or clinical activity at the time of data collection. History of acute severe colitis was seen in 21.2% (10/47) of patients (data was available for only 47 patients out of total 167 patients), while the data regarding steroid refractory/dependent course was not available (Table 1). Only three patients were on biologicals (all three were on infliximab).
Venous thromboembolism
VTE was seen in five patients, with four patients having DVT, two having CVT and one patient having both CVT and DVT. All the patients included in this study were symptomatic for VTE. Patients diagnosed with DVT presented with limb swelling, while one patient with CVT presented with persistent headache. Another patient presented with focal seizures and altered sensorium; subsequent evaluation revealed transverse sinus thrombosis. The data regarding thrombus being acute or chronic was not available. The mean age of patients with VTE was 48 ± 18.75 years and all were females. None of the patients had PTE, arterial thrombosis or abdominal vein thrombosis. All the five patients were started on oral anticoagulants; two received warfarin and three received directly acting oral anticoagulants (DOACs). All patients continued anticoagulation except one who was lost to follow-up. The extent of disease was proctitis and pancolitis in two patients each, and one patient had left sided colitis. The median duration of follow-up was five years, and median duration between IBD diagnosis and occurrence of VTE was three years. Disease activity (clinical or biochemical) was seen in three patients and all patients had mild activity as per SCCAI (Table 2 and 3). Four patients had past history of steroids exposure. Patient 1 (Table 2) had history of steroid intake at the time of diagnosis of disease and she had VTE 48 weeks after the diagnosis of UC. She was having clinical disease activity at the time of VTE. Patient 2 did not receive steroids and was in clinical remission at the time of VTE. Patient 3 developed DVT three years after the diagnosis of UC. She was on mesalamine and had received steroids 2 years prior to VTE. Patient 4 had flare of the disease and was started on intravenous steroids followed by oral steroids. She developed VTE after 3 months, while being on tapering dose of steroids. Patient 5 had both CVT and DVT. Her disease was in remission. She had history of oral steroids at the time of diagnosis of UC (Table 2). The cumulative dose of steroids received by the patient was not available in the records.
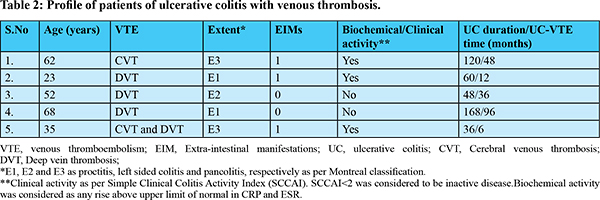
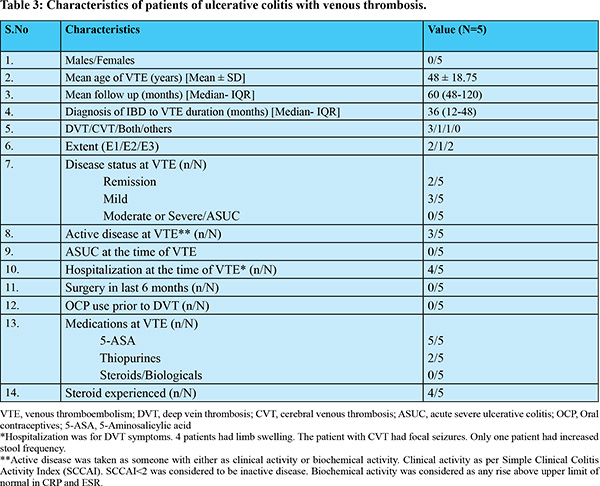
At the time of VTE, all patients were on mesalamine, two were on azathioprine, one was on tapering steroids and none of the five patients were on biologicals. At a median follow-up of five years, there were no deaths reported, and none of the patients required colectomy due to their ulcerative colitis. Procoagulant work-up (Protein C, protein S, antithrombin III, antiphospholipid antibody and homocysteine levels) were available for four patients. All four patients were having normal procoagulant work up. None of the patients of VTE on oral anti-coagulants developed gastrointestinal bleed during the follow-up period and all the five patients had their disease in remission on their medications (Table 2 and 3).
Discussion
This study found a 3% prevalence of VTE in a cohort of UC patients at a single center. Four patients had history of acute severe colitis in past and were treated with steroids. All patients who developed VTE were females.The median duration of VTE development was three years and DVT was the most common type of VTE. During the median follow- up of five years, no adverse outcomes such as the need for colectomy or mortality were observed in patients who experienced VTE. The prevalenceof VTE in IBD has been found to vary from 1% to 6%. A retrospective study from India involving a large cohort of 3500 patients with IBD showed a prevalence of VTE in UC and CD to be 0.85% and 1.2%, respectively.10 Another study from Japan involving 47 hospitalized patients, all of whom underwent ventilation-perfusion scan and magnetic resonance venography or conventional venography found that 17% patients had VTE, emphasizing the occurrence of many asymptomatic cases.23 Autopsy series have shown even a much higher incidence. Papay et al. reported a prevalence of 4.8% in UC patients and other authors have shown a prevalence of up to 7% in UC.2,24 Mccurdy et al. has shown the risk of post-discharge VTE in hospitalized IBD patients at 12 months to be 2.3% and 1.6% for non-surgical and surgical patients, respectively. For ulcerative colitis, VTE incidence in surgical and non-surgical patients was 2.22% and 2.0%, respectively.25 In our study, all cases of VTE were observed in females. Whereas, studies have generally found that there is no significant gender difference in the occurrence of VTE between people with IBD and those without it.15 However, one study has suggested that there may be a slightly higher tendency towards VTE in females with IBD who are between the ages of 20 and 39 years.14 It’s worth noting that this particular study did not include a control group for comparison. However, it’s important to keep in mind that our study had a very small sample size and event rates, so we cannot draw definitive conclusions from this data alone. The risk of VTE has been found to be associated with active disease and flares, particularly more in non-hospitalized patients during flare-ups.10,12,26 The data from India has shown that approximately 95% of patients with VTE had moderately-severe disease at the time of VTE, and roughly 50% of VTE cases occurred during acute severe colitis flares.10 In a recent population based study, Bernstein et al, reported that steroid use, anti-tumour necrosis factor-alfa (TNF-alfa) use of less than 3 years, combined use of steroids and anti-TNF-a, any comorbidities and hospitalization was associated with occurrence of VTE. The incidence was observed to be higher in CD (8.4%) compared to UC (6.9%), while gender did not appear to play a role.24 However, none of our patients had moderately severe or severe disease at the time of VTE, though, four patients of VTE in our study had history of acute severe colitis in past and were treated with steroids. Scocille et al. has shown that two-third of VTE occurs in outpatients and most common VTE is DVT.17 The median duration for the occurrence of VTE after diagnosis of UC in this study was three years, which is longer than what has been reported in other studies.10 Our study had a very few patients on biologicals, which could indicate more patients with milder disease in our cohort or may be due to some part of the study period falling during COVID-pandemic. This again explains lack of colectomy/surgery in our cohort. The increased risk of VTE in IBD is attributed to hypercoagulability, but studies have reported variable results. Acquired factors are thought to play the most significant role in pathogenesis of VTE in IBD. Patients with IBD have thrombocytosis, decreased mean platelet volume and increased platelet reactivity leading to increased thrombotic risk.27,28 Moreover, IBD patients tend to have elevated levels of platelet derived large extracellular vesicles, which can have a bearing via different mechanisms. Additionally, procoagulant factors are known to increase, anticoagulation factors tend to decrease, and fibrinolysis may be impaired in IBD, all of which may contribute to an increased risk of VTE in IBD.27-29 Moreover some authors have recommended post-hospitalization prophylaxis for VTE in patients with IBD. This stems from increased risk of VTE in post-IBD or non-IBD hospitalization for these patients. Additionally, these authors have also suggested administering prophylaxis for at least a month in those deemed to be at high risk (> 5% risk of VTE).30 Our study has some limitations that must be acknowledged. Firstly, our sample size was small, comprising only 167 patients from a single ethnic group and a single centre. We acknowledge that a small sample size and consequent smaller event rate may be the reason we did not find any association with the known risk factors for VTE in IBD. Secondly, because this was a retrospective study, it had some inherent limitations, such as missing data and a lack of information regarding individual factors that predispose patients to VTE. Most of the data was obtained from outpatient cards and included patients with less severe disease course. Furthermore, because we did not have a control population, we could not assess the relative risk of VTE in UC. Hence, we could not compare the risk of VTE in UC with any of the disease states or general population. We did not have complete information on steroid refractory/experienced status, endoscopic disease status, medications, or disease course, all of which may have influenced the probability of VTE. We acknowledge that we have incomplete data on some important variables, and this must be kept in consideration while interpreting the results of this study. These variables are the independent confounders that may limit the external validity of this study. Hence this study could not elucidate an underlying etiology or risk factors for vascular thrombosis in this cohort of UC patients. This study mainly relied on data from outpatient records, so it is possible that the cases that occurred during hospitalization may have been missed. Additionally, we did not investigate genetic or coagulation defects that could have shed light on independent causes of thrombosis in these patients. To summarize, this study sheds light on the prevalence and risk factors for VTE in patients with UC. It also emphasizes the need for studies on outpatients with UC, who may represent a different subset than hospitalized patients.
Conclusion
The prevalence of VTE in this study was found to be 3%, with most episodes occurring in patients with a history of steroid use. Most patients of UC with VTE had history of acute severe colitis in past and history of past steroid use. However, the small sample size could not provide any statistically significant association.
References - Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. 2023 Apr;20(4):248-262.
- Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013 Oct;7(9):723–9.
- Baylis RA, Smith NL, Klarin D, Fukaya E. Epidemiology and Genetics of Venous Thromboembolism and Chronic Venous Disease. Circ Res. 2021 Jun 11;128(12):1988–2002.
- Nguyen GC, Sam J. Rising Prevalence of Venous Thromboembolism and Its Impact on Mortality Among Hospitalized Inflammatory Bowel Disease Patients. Am J Gastroenterol. 2008 Sep;103(9):2272–80.
- Talbot RW, Heppell J, Dozois RR, Beart RW. Vascular Complications of Inflammatory Bowel Disease. Mayo Clin Proc. 1986 Feb;61(2):140–5.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A Population-Based Perspective of the Hospital Incidence and Case-Fatality Rates of Deep Vein Thrombosis and Pulmonary Embolism: The Worcester DVT Study. Arch Intern Med. 1991 May 1;151(5):933–8.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Predictors of Survival After Deep Vein Thrombosis and Pulmonary Embolism: A Population-Based, Cohort Study. Arch Intern Med. 1999 Mar 8;159(5):445.
- Miehsler W. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004 Apr 1;53(4):542–8.
- Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013 May;37(10):953–62.
- S Y, Kedia S, Teja V, Vuyyuru SK, Yadav N, Sahu P, Jain S, Yadav DP, Bopanna S, Mouli VP, Madhu D, Sharma R, Das P, Makharia G, Ahuja V. Thromboembolism is associated with poor prognosis and high mortality in patients with inflammatory bowel disease: A case-control study. Indian J Gastroenterol. 2022 Aug;41(4):325-335.
- Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous Thromboembolism in Inflammatory Bowel Disease. Am J Gastroenterol. 2004 Jan;99(1):97–101.
- Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. The Lancet. 2010 Feb;375(9715):657–63.
- Yoshida H, Granger ND. Inflammatory bowel disease: A paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009 Aug;15(8):1245–55.
- Huerta C. Risk Factors and Short-term Mortality of Venous Thromboembolism Diagnosed in the Primary Care Setting in the United Kingdom. Arch Intern Med. 2007 May 14;167(9):935.
- Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011 Jul 1;60(7):937–43.
- Boccatonda A, Balletta M, Vicari S, Hoxha A, Simioni P, Campello E. The Journey Through the Pathogenesis and Treatment of Venous Thromboembolism in Inflammatory Bowel Diseases: A Narrative Review. Semin Thromb Hemost. 2023 Oct;49(7):744-755.
- Scoville EA, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Venous Thromboembolism in Patients with Inflammatory Bowel Diseases: A Case-control Study of Risk Factors. Inflamm Bowel Dis. 2014 Apr;20(4):631–6.
- Stegeman B H, de Bastos M, Rosendaal F R, van Hylckama Vlieg A, Helmerhorst F M, Stijnen T et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis BMJ 2013; 347 :f5298.
- Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016 Mar 1;10(3):239–54.
- Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019 Feb 1;13(2):144-164.
- Walmsley RS, Ayres RCS, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998 Jul 1;43(1):29–32.
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017 Jun 1;11(6):649–70.
- Sonoda K, Ikeda S, Mizuta Y, Miyahara Y, Kohno S. Evaluation of venous thromboembolism and coagulation-fibrinolysis markers in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2004 Oct;39(10):948–54.
- Bernstein CN, Nugent Z, Singh H. Persistently High Rate of Venous Thromboembolic Disease in Inflammatory Bowel Disease: A Population-Based Study. Am J Gastroenterol. 2021 Jul;116(7):1476–84.
- McCurdy JD, Kuenzig ME, Smith G, Spruin S, Murthy SK, Carrier M, et al. Risk of Venous Thromboembolism After Hospital Discharge in Patients With Inflammatory Bowel Disease: A Population-based Study. Inflamm Bowel Dis. 2020 Oct 23;26(11):1761–8.
- Heo CM, Kim TJ, Kim ER, Hong SN, Chang DK, Yang M, et al. Risk of venous thromboembolism in Asian patients with inflammatory bowel disease: a nationwide cohort study. Sci Rep. 2021 Jan 21;11(1):2025.
- Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease: Vascular disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013 Jul;28(7):1095–113.
- Lagrange J, Lacolley P, Wahl D, Peyrin-Biroulet L, Regnault V. Shedding Light on Hemostasis in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2021 Jun;19(6):1088-1097.e6.
- Senchenkova E, Seifert H, Granger D. Hypercoagulability and Platelet Abnormalities in Inflammatory Bowel Disease. Semin Thromb Hemost. 2015 Aug 13;41(06):582–9.
- Murthy SK, Robertson McCurdy AB, Carrier M, McCurdy JD. Venous thromboembolic events in inflammatory bowel diseases: A review of current evidence and guidance on risk in the post-hospitalization setting. Thromb Res. 2020 Oct;194:26–32.
|
|
|
 |
|
|