Introduction
Hepatotrophic viruses such as hepatitis A, B, C, D, and E (together known as hepatitis A through E viruses) are the most common cause of viral hepatitis worldwide. These hepatotropic viruses account for more than 95% of all viral hepatitis, and the clinical and laboratory features are well understood. Furthermore, blood ELISA tests, which are readily and widely available, can be used to diagnose hepatitis A through E viruses.
Overall the incidence for hepatitis A-E has remained stable although the disease associated life years (DALY) have declined.1,2 However, the incidence of hepatitis from unusual or non-hepatotropic viruses are likely increasing as exemplified by dengue hepatitis in tropical countries.3,4 EBV in some areas of the United Kingdom,5 and adenoma associated virus type 2 in children in Western countries.6 In studies involving more than 250 patients each from 2 centers from India, 10-18.5% were caused by non-hepatotropic viruses.7,8 The hepatitis caused by these viruses may sometimes be severe enough to cause acute liver injury or failure as listed in Table 1. The goal of this review is to provide an overview of the available data on atypical viral hepatitis caused by 4 viruses namely Dengue virus, Epstein-Barr virus, Cytomegalovirus and Varicella Zoster virus in an effort to promote early detection, initiate appropriate treatment, and reduce morbidity and mortality.
Dengue Virus Hepatitis
Dengue is an arboviral disease caused by Dengue virus (DENV) which belongs to the Flavivirus genus of the Flaviviridae family. DENV can be subclassified into four serotypes, DENV-1, DENV-2, DENV-3 and DENV-4. All 4 serotypes are associated with hepatitis and ALF. Dengue is transmitted in a majority of patients by the bite of the infected Aedes aegypti (A. aegypti) mosquito. Bite from A. albopictus is responsible in a small number of patients.
It is estimated that 100-400 million dengue infections occur each year with about half of the world’s population at risk of dengue.3 The regions that are most affected include the South-East Asian region, the Americas, and the Western Pacific regions; about 70% of the global burden is reported from Asia.9 With an increase in international travel, a person can acquire the infection in an endemic area and develop disease in countries where the disease is uncommon.
The symptoms of DENV infection can vary from asymptomatic infection to mild symptomatic dengue fever to severe life-threateningfeatures of dengue hemorrhagic fever and dengue shock syndrome. A majority of infected individuals with dengue have mild or no symptom with fever and body aches being the predominant symptoms.
The liver is commonly involved in DENV and can vary from no or minimal elevation of aminotransferases to severe injury characterized by massive rise in transaminases > 1000 IU and rare progression to acute liver failure. Aminotransferases elevation is reported in 60% to 97% of patients.10 The WHO lists transaminase elevation >1000 IU/l, hepatomegaly and ascites as warning signs of dengue infection indicating severe disease.11 Presence of hyperbilirubinemia or jaundice is less common.
Pathogenesis of liver injury in DENV infection.
Although the liver is involved in a large number of patients, the pathogenesis is still not clear. The following are some of the hypotheses ascribed for hepatic involvement.
1. Direct cytopathic effects of the virus as suggested by the identification of DENV within hepatocytes and Kupffer cells.12
2. Host immune response on the liver cell. This is explained by the occurrence of severe illness and liver injury during the second bout of infection by a mechanism known as antibody-dependent enhancement of disease, wherein a specific range of antibody during second infection triggers severe disease.13
3. Apoptosis
4. Micro vesicular steatosis or macrovesicular steatosis.14
4. Circulatory compromise leading to metabolic acidosis.
5. Hypotension leading to hypoxic or ischemic hepatitis.
5. Localized vascular leakage or sinusoidal obstruction inside the liver without peripheral signs of hypotension.15,16
6. Rhabdomyolysis caused by the pathophysiology of the disease leading to profound rise in AST than ALT. In such a scenario, the absolute rise in AST may not be a reflection of hepatic dysfunction.17
Acute liver failure (ALF) like presentation is a rare complication of dengue, occurring in 0.3% to 0.7% of patients.4,18 It occurs in the afebrile or defervescence (recovery) phase of the disease. ALF may present with catastrophic suddenness. In one large series from India, of the 10,108 patients diagnosed with DENV from 2014 to 2017, 36 cases (0.35%) exhibited features of ALF, with 72% (n=26) and 28% (n=10) presenting with features of hyperacute and acute liver failure, respectively.4 An earlier study from Thailand identified 6 cases of ALF among 1926 (0.3%) dengue cases.18
In patients with severe dengue, aminotransferase elevation is related to the severity of illness; however, this finding does not distinguish between survivors and non survivors.4,19 Surprisingly, neither the degree of bilirubinemia nor the presence of coagulopathy distinguishes between survivors and non-survivors, despite the fact that the levels are higher in non-survivors. Because dengue is primarily a systemic disease with liver involvement, other features of systemic involvement such as admission acidemia (Ph), serum lactate, and hemoglobin were found to predict mortality.20 When compared to ALF caused by hepatotropic viruses, there are certain distinctive features that can help differentiate between the two as shown in Table 2.
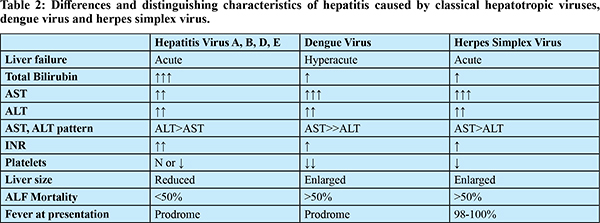
AST and ALT can rarely reach levels >20000 U/L with a relatively mild rise in bilirubin and is one of the hallmarks of ALF due to dengue. The cause for such an astronomical rise in transaminase is ascribed to ischemic hepatitis caused by capillary leak. In others without shock, this could be due to liver sinusoidal microcirculatory dysfunction leading to hepatic ischemia. The reversed portal blood flow and portal hypertension may result from the increased sinusoidal pressure.15 The massive rise in transaminases may also be due to an extrahepatic source such as muscle necrosis or rhabdomyolysis.17
Although not considered a hepatotropic virus, in some cases of dengue hepatitis, viral particles are seen by immunohistochemistry in liver tissue raising concern that in some cases, DENV may be responsible for causing hepatitis, necrosis and steatosis.21 Unlike prototypical viral hepatitis that is characterized by hepatocyte necrosis and inflammation, there is generally a lack of substantial necrosis and inflammatory infiltrates in dengue hepatitis in most cases. Instead minimal to modest microvesicular or macrovesicular steatosis, and apoptosis is seen suggesting injury to the mitochondrial machinery.22
Overall children appear to develop a more severe disease than adults, with hemophagocytic lymphohistiocytosis (HLH) reported more often in children. HLH can complicate the natural history of DENV infection and should be suspected in the presence of prolonged fever, worsening disease status with cytopenia, hepatosplenomegaly and the diagnosis confirmed by a bone marrow aspiration/biopsy.23
Diagnosis is easily established by NS1 antigen test and IgM antibody against DENV. Treatment is largely supportive. Patients with severe disease need to be transferred or admitted in advanced facilities. The key to reducing morbidity and death is maintaining hydration, systemic pressure, and supporting organ failure when it happens. Although N-acetyl cysteine has been used in patients with ALF, its role specifically in patients with dengue ALF is lacking.
Herpes Simplex Virus Hepatitis
HSV hepatitis is caused by both HSV 1 and HSV 2, which belong to the herpesviridae family. HSV 2 is more common than HSV 1 but does not have distinctive features.24 Infection can occur in all age groups in immune suppressed and immune competent individuals, but more commonly in the former. HSV hepatitis may occur as a part of primary infection or a result of reactivation brought on by “stressors” such as pregnancy, or immune suppression. Since HSV hepatitis is uncommon and lacks recognizable symptoms, it can be difficult to diagnose and frequently goes undiagnosed.
Hepatitis symptoms can range from a slight, asymptomatic increase in transaminases to characteristics similar to severe viral hepatitis and ALF. Without a significant grade of encephalopathy, the clinical state can change from acute hepatitis to ALF quickly. ALF presentations are uncommon, making about 0.7% and 1.4% of all ALF cases in the American and French series, respectively. HSV makes up 8.7% (in America) and 4.7% (in France) of the viral causes of ALF. In one review of 137 reported cases, the breakup of infection in each category was 24% in immune competent, 23% in pregnancy, 30% in transplant immunosuppressed individuals, and 30% in non-transplant immunosuppressed persons. At least 23% of reported instances of HSV occur in pregnancy where it is confused for pregnancy specific liver diseases such as acute fatty liver of pregnancy (AFLP) or hemolysis elevated liver enzyme and low platelet (HELLP) syndrome. As a result, the diagnosis of HSV hepatitis is suspected when the liver condition does not improve following delivery. The outcome is poor, but this is because of the inability to link the subtle clues that accompany the disease resulting in delayed diagnosis. Only 23% of cases are diagnosed antemortem.25
The diagnosis of HSV is made by positive serum HSV DNA by PCR, anti-HSV immunoglobulin M, a liver biopsy that depicts intranuclear inclusion bodies and a positive immunohistochemistry to HSV antigen.
Once suspected clinically, or in cases of severe hepatitis or ALF on unknown etiology, empiric acyclovir therapy should be considered without waiting for confirmation of the disease or until HSV confirmatory tests return negative.25 In a review of published cases, mortality was 88% without treatment with acyclovir compared with 51% with acyclovir,25 underpinning the importance of timely treatment of this condition. Variables associated with poor outcome include: male sex, age over 40 years, immunocompromised state, ALT over 5000 IU/l, thrombocytopenia, coagulopathy, encephalopathy and absence of antiviral therapy.25
Clinical clues for early diagnosis and treatment:
1. Absence of common causes of acute hepatitis or acute liver failure.
2. Presence of fever. Fever is present almost universally.
3. In an appropriate clinical setting (for example pregnancy, immunosuppressive state), the presence of leukopenia, massive rise in transaminases (sometimes several hundred-fold above normal), and mild hyperbilirubinemia, (anicteric hepatitis but not in all cases) should arouse a suspicion of HSV hepatitis.
4. AST >>ALT (Ratio >2:1)
5. Ulceration or mucocutaneous vesicles in the oropharynx or genitalia. In these situations, a Tzanck smear can be performed at the patient’s bedside, and the results can be obtained quickly to help with management.
6. Liver biopsy-intranuclear inclusion bodies with minimal inflammatory response and spotty necrosis.
Epstein Barr Virus Hepatitis
Epstein-Barr Virus (EBV) is a member of the herpes family with a predilection for B cells of the oropharynx. It spreads through oral secretions and causes primary infection in more than 90% of the adult population. Primary infection elicits a strong cellular immune response and persists in the resting memory B cells with capability for indefinite growth.26 EBV hepatitis is hypothesized to be due to either direct damage to the hepatocytes or to indirect immune mediated damage to the liver.27
Primary EBV infection in children and adolescents is usually asymptomatic and as a part of infectious mononucleosis may be associated with two-to three fold elevation in liver enzymes in 80-90% of patients.28 Transaminases are rarely 10-15 fold elevated, but levels >1000 IU/L that are seen in conventional hepatitis A-E is uncommon.27 Jaundice is seen <5% cases,28 with a cholestatic biochemical picture seen in about 65 % of cases29 particularly in individuals >40 years old.5
But in patients who progress to ALF, high enzymes have been reported such as the 2 patients in the acute liver failure study group (ALFSG) with AST and ALT >1000 IU/L. Severe hepatitis or acute liver failure requiring liver transplantation was reported in 0.21% of acute liver failure cases in the United States with a high fatality rate.30 EBV is implicated in 60-85% cases of post-transplant lymphoproliferative disorders (PTLD), a few of them involving the liver allograft, presumably due to EBV driven B cell proliferation.31 Unexplained hepatitis in immunosuppressed individuals should raise the suspicion for EBV infection.27
Heterophile and EBV-specific antibodies that forms the basis of the Monospot and Paul Bunnell tests are used to screen patients for infectious mononucleosis. Viral capsid antigen (VCA), EBV nuclear antigen (EBNA) and early antigens are other tests required for confirmation. IgM antibodies are not detected in association with chronic infection, so their presence is virtually diagnostic of primary EBV infection.32
Although EBV hepatitis exhibits a wide spectrum of histologic patterns, in select cases, liver biopsy may be indicated to establish an etiological diagnosis. Moderate to marked lymphocytic infiltrate in the portal tracts with a beaded sinusoidal lymphocytic infiltration seen in a single file pattern is characteristic of EBV hepatitis.33 Ancillary tests such as in situ hybridization and polymerase chain reaction (PCR) are often required for the confirmation of diagnosis.
Treatment of EBV infection is supportive as the infection usually resolves in 2-4 weeks. There are no proven benefits with corticosteroids or acyclovir.34 For those that develop acute liver failure1 liver transplantation may be needed. Reduction of immunosuppression is advised once PTLD is diagnosed.
Cytomegalovirus Hepatitis
Cytomegalovirus (CMV) is a double stranded DNA virus that belongs to the herpesviridae family. The liver gets infected during primary infection through hematogenous spread of virus or following reactivation during immunosuppression.35 CMV liver injury is caused by both direct viral mediated cytopathy and indirectly by released cytokines and cytotoxic T-lymphocyte (CD8+) mediated cytotoxicity.36,37
In immunocompetent individuals, primary CMV infection is usually asymptomatic or may present with non-specific symptoms such as fever, malaise, myalgia, abdominal pain, hepatosplenomegaly, and lymphadenopathy.38 Severe hepatic dysfunction is very uncommon in immunocompetent individuals. In immunosuppressed individuals such as patients with HIV infection, post organ transplantation, or individuals receiving immunosuppressive drugs, CMV hepatitis is due to reactivation.39,40 In such cases, severity may vary from mild to severe and often is associated with features of disseminated CMV infection rather than CMV hepatitis alone.40 In post liver transplant recipients, CMV hepatitis is commonly observed in the first three months after transplantation;the incidence varies from 2.1% to 29%.41 CMV increases the risk of both acute and chronic allograft rejection.40 CMV reactivation and subsequent development of CMV hepatitis is also observed in patients with drug induced hypersensitivity skin reactions and recently following COVID-19 infection.42,43
CMV hepatitis causes elevation of aminotransferases, the level of which differs among immunocompetent and immunosuppressed hosts. Due to robust immune response in immunocompetent individuals, aminotransferase elevation is seen up to ten times with modest raise in bilirubin (up to 5-9 mg/dl), whereas in immunosuppressed aminotransferases elevation is up to 2 fold along with 1-10 times elevation of alkaline phosphatase and 2-30-fold elevation of gamma-glutamyl transferase.41,44
The diagnosis of CMV hepatitis is established based on clinical suspicion in appropriate clinical setting by CMV serology, CMV-DNA PCR assay and liver histology findings that include enlargement of portal triads, portal and periportal mononuclear cell infiltrates consisting of lymphocytes, plasma cells, focal hepatocyte necrosis, enlargement of cells, and characteristic viral inclusion bodies (owl’s-eye). Occasionally giant cell granuloma and micro abscess can be observed.45-47 Immunohistochemistry against CMV antigen in biopsy tissue increases the diagnostic yield.
When to Suspect CMV Hepatitis?
CMV hepatitis needs to be suspected in the following clinical situations:
1. In patients with post organ transplantation who present with clinical features of fever, myalgia, malaise, cytopenia and liver chemistry abnormalities.
2. In post liver transplantation patients who present with features of acute rejection and cytopenia.
3. In patients with drug induced hypersensitivity skin reaction/ drug reaction with eosinophilia and systemic symptoms (DRESS) who develop liver chemistry abnormalities with or without features of systemic viremia.
4. Liver chemistry abnormalities with systemic features of CMV viremia in patients receiving immunosuppressive drugs especially anti TNF–a medications, steroids, and other immunosuppressive drugs.
5. Rarely in immunocompetent individuals who present as pyrexia of unknown origin with liver chemistry abnormalities.
A majority of immunocompetent patients with CMV hepatitis improve spontaneously without treatment. Those with persistent severe symptoms due to target organ damage and those with CMV hepatitis in immunosuppressed conditions require anti-viral drug therapy48 such as intravenous ganciclovir 5 mg/kg twice a day or oral valganciclovir 900 mg twice a day for three weeks. Treatment response needs to be monitored by measuring CMV DNA viral load. Treatment can be discontinued when viral eradication is achieved on repeat CMV PCR test at least on one or two occasions.49
Varicella Zoster Virus
Varicella zoster virus (VZV) is a double-stranded DNA virus and a member of the Herpesviridae family. Primary infection with VZV produces a generalized vesicular rash called chickenpox, while reactivation later in life causes zoster or shingles. Following primary infection, VZV remains dormant in the dorsal root ganglia and is reactivated when the cellular immunity is impaired as in older individuals and those who are immunosuppressed.
Although visceral involvement can occur in primary disease, it is more common in reactivation and involves lungs (interstitial pneumonitis), heart (myocarditis), pancreas (pancreatitis), and brain (meningoencephalitis). Liver involvement by VZV can occur in both primary infection and reactivation disease and generally as a part of multisystem involvement. Liver involvement ranges from mild hepatitis to acute liver failure which although rare is frequently fatal.50-52 Elevation in liver enzymes can be seen in up to 3.4% of the children with chickenpox with significant hepatitis being reported only rarely.50 Reactivation of VZV is characterized by typical herpetic vesicular skin lesions in a dermatomal distribution; however, VZV infection can occur without cutaneous lesions particularly in those with bone marrow or solid organ transplant recipients leading to delay in timely diagnosis.50 Varicella hepatitis in immunocompetent hosts is usually self-limiting and asymptomatic with subclinical elevation in serum transaminase levels.52 History of contact with patients with chicken pox is helpful in early diagnosis and prompt treatment initiation. Review of case reports and small case series in literature shows that AST and ALT elevation is usually 2-5 times the upper limit of normal; however, in fulminant hepatic failure (FHF), the enzymes are >10 fold above normal.51,52 Disseminated intravascular coagulation along with gastrointestinal bleed have also been reported in patients presenting with FHF.53
Definitive diagnosis of VZV hepatitis is by liver biopsy, histopathologic examination showing typical inclusion bodies, culture, and VZV PCR. VZV PCR is the most sensitive method for confirming the diagnosis of varicella in skin lesions. IgM serology provides evidence for a recent active VZV infection but cannot discriminate between a primary infection and reinfection or reactivation from latency since specific IgM antibodies are transiently produced on each exposure to VZV. IgM tests are also inherently prone to poor specificity.
Prompt systemic antiviral treatment with acyclovir, valacyclovir, or famciclovir, is indicated as first-line treatment for immune-compromised patients and immunocompetent patients with a severe clinical course. Standard anti-viral dosage regimens for non-immunocompromised patients includes valacyclovir 1000 mg 3 times a day for 7 days or acyclovir 800 mg 5 times a day for 7-10 days.51,54 Prophylaxis with varicella zoster immunoglobulin (VZIG) is recommended for seronegative patients who are susceptible to infection following virus exposure.
COVID Associated Hepatitis
Severe acute respiratory syndrome due to coronavirus disease (COVID) was first identified in late 2019 in Wuhan, China. Primarily COVID-19 infection causes respiratory symptoms; however, it is known to cause various extra-pulmonary manifestations including liver involvement. The incidence of hepatic manifestations varies from 14.8%-53%.55
Pathophysiology: Multiple factors contribute to hepatic involvement in COVID-19 as summarized below-
a) Direct viral cytotoxicity: COVID-19 virus gains entry into the cells through ACE2 receptors. In liver ACE-2 receptors are predominantly expressed in cholangiocytes and in small numbers of hepatocytes, Kupffer cells and endothelial cells.56 Upon binding of COVID-19 virus with ACE-2 receptors, ACE-2 is endocytosed which reduces the surface receptors. Viral particles cause direct cytotoxicity by interfering with lipid metabolism.55,56
b) Immune mediated effects: A subset of patients with COVID-19 develop exaggerated inflammatory response characterized by elevated levels of various cytokines like interleukin (IL)-2, IL-6, IL-7, IL-10, tumour necrosis factor (TNF)- alpha and granulocyte colony stimulating factor.56 The exaggerated inflammatory response may lead to hypotension, hypoxia, endothelial dysfunction and microthrombi which in turn causes liver damage.57
c) Hypoxia: Hypoxic injury to liver occurs due to systemic hypoxia secondary to acute respiratory distress-syndrome (ARDS) in severe COVID-19 infection, hepatic vascular thrombosis and also due to right heart failure associated with myocardial dysfunction.58
d) Drug induced liver injury: Various drugs used in the treatment of COVID-19 infection can cause liver injury such as remdesivir, favipiravir, tocilizumab, high dose of steroids and acetaminophen in patients with underlying liver disease.59
e) Gut microbiota: Severe COVID-19 infection is associated with dysbiosis of gut microbiota. The disruption of gut microbiota leads to bacterial translocation and endotoxemia which in tern leads to hepatic injury.60
f) Mitochondrial damage: Mitochondrial abnormalities are seen in liver specimens in patients with COVID-19 infection which is implicated in pathogenesis of steatohepatitis associated with COVID-19 infection.61
Liver Histology: The morphological changes in liver are described mainly in autopsy studies.62 The histological changes seen include micro and macro vesicular steatosis, mild lobular inflammation, cholestasis and lobular apoptosis.
Presentation: The predominant hepatic manifestation in majority of patients with COVID-19 infection, is mild to moderate liver biochemistry abnormalities.63 In known patients with cirrhosis/ autoimmune hepatitis, COVID-19 may cause exacerbation of underlying liver disease. A subset of children develop multisystem inflammatory syndrome following COVID-19 infection due to persistent activation of immune system. Liver injury in the form of massive raise in liver enzymes, jaundice and coagulopathy occurs as part of multisystem injury.64
Management: The reasons for liver biochemistry abnormalities seen in COVID-19 are multifactorial. Apart from COVID-19 infection, other causes like infection due to hepatotropic virus infection (hepatitis A, E, B and C), myositis, drug induced liver injury, multisystem inflammatory syndrome and underlying chronic liver disease need to be ruled out by appropriate investigations. Hepatic injury associated with COVID-19 needs supportive care and antiviral drugs against COVID-19 in subset of patients. COVID-19 infection in patients with underlying decompensated cirrhosis increases mortality.
Indeterminate Hepatitis in Children
Reports of a sudden and unexpected surge in the onset of cryptogenic acute hepatitis, acute liver injury and liver failure in previously healthy children surfaced in the United Kingdom in 2022. These were patients in whom usual causes of hepatitis such as autoimmune hepatitis, drug induced hepatitis and hepatitis A to E were excluded. Since the issuance of an alert by the WHO which triggered global case finding1 more than 1000 cases of probable unknown hepatitis have been reported in children from across 35 countries. The WHO working case definition of a probable case of indeterminate hepatitis includes any child 16 years or younger presenting with non-hepatitis A-E with serum transaminases >500 IU/L (AST or ALT) since 1 October 2021. Most common symptoms reported include vomiting, jaundice, malaise and abdominal pain. While most patients recovered uneventfully with conservative management, 45 children have required liver transplantation and 18 deaths reported.65 Besides supportive management1 case reports of treatment using corticosteroids suspecting immune dysregulation and intravenous cidofovir have also been published.66
Studies published thence from the United Kingdom and the USA have linked the indeterminate hepatitis to adenovirus (AdV) which was detected in the blood and or liver tissue in up to 68% cases in one series with most (81%) belonging to the genotype Ad41F.67 Adenovirus is a double stranded DNA virus known to cause self-limited conjunctivitis, respiratory infection and gastroenteritis in immunocompetent individuals and does not usually cause hepatitis in immunocompetent individuals. Metagenomic investigations of UK patients detected higher concentrations of adeno associated virus (AAV) type 2 (AAV2) in liver and body fluids than in controls.6 AAV2 needs the help of adenovirus as a helper virus to complete its lytic replication cycle. It is postulated that adenovirus acts as a trigger with cofactor such as AAV2 and causes liver injury.
Amongst the causal hypothesis postulated is the fact that risk–naïve children were suddenly exposed to a host of viruses after lockdown restrictions were relaxed increasing their susceptibility to otherwise innocuous viruses. Their young age made it unlikely that they had prior exposure to these viruses and a reduction in maternal immunity at 18 months of age put them further at risk. This explanation is underscored by the young age (median age 3 years) and the absence of COVID-19 vaccine in the pediatric age group. Moreover some had co-infections with cytomegalovirus, Epstein-Barr virus and enterovirus besides adenovirus.68 Microbiological examination comparing pediatric respiratory samples pre and post pandemic have also confirmed increased incidence of AAV2 in patients post COVID, hence cementing the fact that there are a greater number of children unexposed to AAV2, leading to greater spread of the virus once distancing restrictions were lifted.69 The absence of adenovirus in explant livers make direct viral cytotoxicity unlikely and instead causes liver damage presumably through immunological mechanisms.
With reported cases now waning off since the initial days post pandemic, the argument that the 2022 outbreak was an epidemiological phenomenon of a pre-existing disease is further strengthened.70 It is still not known whether human adenovirus infection alone or in concert with hitherto unknown cofactors may cause hepatitis in children. It is also likely that an absence of prior exposure to other viruses in childhood during the restrictive phase of COVID-19 pandemic, may have given rise to aberrant immune response leading to hepatitis.71
References
- Devarbhavi H, Asrani SK, Arab JP, et al. Global burden of Liver Disease: 2023 Update. J Hepatol 2023;79:516-537.
- Zeng DY, Li JM, Lin S, et al. Global burden of acute viral hepatitis and its association with socioeconomic development status, 1990-2019. J Hepatol 2021;75:547-556.
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013;496:504-7.
- Devarbhavi H, Ganga D, Menon M, et al. Dengue hepatitis with acute liver failure: Clinical, biochemical, histopathological characteristics and predictors of outcome. J Gastroenterol Hepatol 2020;35:1223-1228.
- Vine LJ, Shepherd K, Hunter JG, et al. Characteristics of Epstein-Barr virus hepatitis among patients with jaundice or acute hepatitis. Aliment Pharmacol Ther 2012;36:16-21.
- Servellita V, Sotomayor Gonzalez A, Lamson DM, et al. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature 2023;617:574-580.
- Chadha MS, Walimbe AM, Chobe LP, et al. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978-81 and 1994-97. Indian J Gastroenterol 2003;22:11-5.
- Gupta E, Ballani N, Kumar M, et al. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol 2015;34:448-52.
- World Health Organization. Dengue and Severe Dengue, Fact Sheets, World Health Organization (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/ Dengue-and-severe-Dengue. Accessed 10 April, 2023.
- Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg 2006;100:608-14.
- Guzman MG, Gubler DJ, Izquierdo A, et al. Dengue infection. Nature Reviews Disease Primers 2016;2:16055.
- Huerre MR, Lan NT, Marianneau P, et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch 2001;438:107-15.
- Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017;358:929-932.
- Sam SS, Omar SF, Teoh BT, et al. Review of Dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl Trop Dis 2013;7:e2194.
- Khongphatthanayothin A, Lertsapcharoen P, Supachokchaiwattana P, et al. Hepatosplanchnic circulatory dysfunction in acute hepatic infection: the case of dengue hemorrhagic fever. Shock 2005;24:407-11.
- Devarbhavi H. Letter to the Editor: Response to “Liver Transplantation for Acute Liver Failure Due to Dengue Fever”. Hepatology 2020;71:2171-2172.
- Devarbhavi H, Paryani B, Singh R. Severe dengue hepatitis with elevated transaminases: is rhabdomyolysis the clue to extreme transaminases elevation? . Hepatology 2016;64:321A.
- Kye Mon K, Nontprasert A, Kittitrakul C, et al. Incidence and Clinical Outcome of Acute Liver Failure Caused by Dengue in a Hospital for Tropical Diseases, Thailand. Am J Trop Med Hyg 2016;95:1338-1344.
- Lee LK, Gan VC, Lee VJ, et al. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl Trop Dis 2012;6:e1676.
- Thanachartwet V, Oer-Areemitr N, Chamnanchanunt S, et al. Identification of clinical factors associated with severe dengue among Thai adults: a prospective study. BMC Infect Dis 2015;15:420.
- de Macedo FC, Nicol AF, Cooper LD, et al. Histologic, viral, and molecular correlates of dengue fever infection of the liver using highly sensitive immunohistochemistry. Diagn Mol Pathol 2006;15:223-8.
- Kularatne SA, Imbulpitiya IV, Abeysekera RA, et al. Extensive haemorrhagic necrosis of liver is an unpredictable fatal complication in dengue infection: a postmortem study. BMC Infect Dis 2014;14:141.
- Raju S, Kalyanaraman S, Swaminathan K, et al. Hemophagocytic lymphohistiocytosis syndrome in Dengue hemorrhagic fever. Indian J Pediatr 2014;81:1381-3.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 2007;13:1428-34.
- Kaufman B, Gandhi SA, Louie E, et al. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis 1997;24:334-8.
- Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343:481-92.
- Bunchorntavakul C, Reddy KR. Epstein-Barr Virus and Cytomegalovirus Infections of the Liver. Gastroenterol Clin North Am 2020;49:331-346.
- Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J 2006;99:544-7.
- Kofteridis DP, Koulentaki M, Valachis A, et al. Epstein Barr virus hepatitis. Eur J Intern Med 2011;22:73-6.
- Mellinger JL, Rossaro L, Naugler WE, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci 2014;59:1630-7.
- Kremers WK, Devarbhavi HC, Wiesner RH, et al. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant 2006;6:1017-24.
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med 2010;362:1993-2000.
- Suh N, Liapis H, Misdraji J, et al. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol 2007;31:1403-9.
- Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis 1999;31:543-7.
- Powers C, DeFilippis V, Malouli D, et al. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol 2008;325:333-59.
- Pape GR, Rieber EP, Eisenburg J, et al. Involvement of the cytotoxic/suppressor T-cell subset in liver tissue injury of patients with acute and chronic liver diseases. Gastroenterology 1983;85:657-62.
- Griffiths PD, Grundy JE. The status of CMV as a human pathogen. Epidemiol Infect 1988;100:1-15.
- Ten Napel CH, Houthoff HJ, The TH. Cytomegalovirus hepatitis in normal and immune compromised hosts. Liver 1984;4:184-94.
- Cheung TW, Teich SA. Cytomegalovirus infection in patients with HIV infection. Mt Sinai J Med 1999;66:113-24.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:93-106.
- Paya CV, Hermans PE, Wiesner RH, et al. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis 1989;160:752-8.
- Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpesvirus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis 2010;10:49.
- Gatto I, Biagioni E, Coloretti I, et al. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: risk factors and impact on mortality. Intensive Care Med 2022;48:706-713.
- Seehofer D, Rayes N, Tullius SG, et al. CMV hepatitis after liver transplantation: incidence, clinical course, and long-term follow-up. Liver Transpl 2002;8:1138-46.
- Snover DC, Horwitz CA. Liver disease in cytomegalovirus mononucleosis: a light microscopical and immunoperoxidase study of six cases. Hepatology 1984;4:408-12.
- Drebber U, Kasper HU, Kern M, et al. Comparison of the lymphocytic infiltrate in Epstein-Barr Virus (EBV) and Cytomegalovirus (CMV) induced hepatitis. Pathology - Research and Practice 2004;200:307.
- Bonkowsky HL, Lee RV, Klatskin G. Acute granulomatous hepatitis. Occurrence in cytomegalovirus mononucleosis. JAMA 1975;233:1284-8.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 2003;37:1603-6.
- Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013;96:333-60.
- Feldman S, Crout JD, Andrew ME. Incidence and natural history of chemically defined varicella-zoster virus hepatitis in children and adolescents. Scand J Infect Dis 1997;29:33-6.
- Mantadakis E, Anagnostatou N, Danilatou V, et al. Fulminant hepatitis due to varicella zoster virus in a girl with acute lymphoblastic leukemia in remission: report of a case and review. J Pediatr Hematol Oncol 2005;27:551-3.
- Anderson DR, Schwartz J, Hunter NJ, et al. Varicella hepatitis: a fatal case in a previously healthy, immunocompetent adult. Report of a case, autopsy, and review of the literature. Arch Intern Med 1994;154:2101-6.
- Pishvaian AC, Bahrain M, Lewis JH. Fatal varicella-zoster hepatitis presenting with severe abdominal pain: a case report and review of the literature. Dig Dis Sci 2006;51:1221-5.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007;44 Suppl 1:S1-26.
- Nardo AD, Schneeweiss-Gleixner M, Bakail M, et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver International 2021;41:20-32.
- Li J, Fan J-G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Trans Hepatol 2020;8:13.
- Abeysuriya V, Seneviratne SL, de Silva AP, et al. Combination of cycle threshold time, absolute lymphocyte count and neutrophil: lymphocyte ratio is predictive of hypoxia in patients with SARS-CoV-2 infection. Trans R Soc Trop Med Hyg 2022;116:628-635.
- Bertolini A, van de Peppel IP, Bodewes FA, et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology (Baltimore, Md.) 2020;72:1864.
- Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy: J Hum Pharm Drug Ther 2020;40:416-437.
- Yeoh YK, Zuo T, Lui GC-Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698-706.
- Miller B, Silverstein A, Flores M, et al. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Scientific reports 2021;11:3.
- Barton LM, Duval EJ, Stroberg E, et al. Covid-19 autopsies, oklahoma, USA. Am J Clin Path 2020;153:725-733.
- Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gast Hep 2020;18:1561-1566.
- Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074-1087.
- Elsheikh R, Tien HT, Makram AM, et al. Acute hepatitis of unknown origin in children: Behind the statistics. Hepatology 2023;77:2118-2127.
- Gutierrez Sanchez LH, Shiau H, Baker JM, et al. A case series of children with acute hepatitis and human adenovirus infection. N Engl J Med 2022;387:620-630.
- Mandal S, Simmons R, Ireland G, et al. Paediatric acute hepatitis of unknown aetiology: a national investigation and adenoviraemia case-control study in the UK. The Lancet Child & Adolescent Health 2023;7:786-796.
- Kelly DA, Stamataki Z. Sudden onset hepatitis in children. Nat Rev Gast Hep 2022;19:553-554.
- Gates S, Andreani J, Dewar R, et al. Postpandemic rebound of adeno-associated virus type 2 (AAV2) infections temporally associated with an outbreak of unexplained severe acute hepatitis in children in the United Kingdom. J Med VIrol 2023;95:e28921.
- Jagadisan B, Verma A, Deheragoda M, et al. Outbreak of indeterminate acute liver failure in children with adenoviraemia–Not a new disease. J Hepatol 2023;79:43-49.
- Jagadisan B, Dhawan A. Outbreak of indeterminate acute hepatitis in children, not a new disease but an epidemiological phenomenon. Hepatology 2023:10.1097.