Leiomyomas are benign tumours of smooth muscle origin. The uterus is the most common site for these benign tumours and is seen in 20-30% of females above 35 years of age1. Unusual growth patterns may be seen in intravenous leiomyomatosis (IVL), benign metastasizing leiomyoma (BML), retroperitoneal leiomyomas and disseminated leiomyomatosis peritonealis disseminata (LPD). LPD are vascular leiomyomas growing along the submesothelial tissues of the abdomino-pelvic peritoneum, usually in females of the reproductive age group. LPD is a rare benign disease and is often misdiagnosed as disseminated malignancy or peritoneal carcinomatosis. There are about 150 cases reported till date2. We are describing a 28-year-old female who was diagnosed as a case of leiomyomatosis peritonealis disseminata and was treated by surgical excision.
Case Report
A 28-year-old lady presented with complaints of awareness of a lump right upper abdomen for the last 2 years, which was progressively increasing in size with pain abdomen on and off for the last 6 months. She had undergone laparoscopic uterine myomectomy 7 years back (biopsy suggestive of leiomyoma). There was no history of altered bowel habit, vomiting, jaundice, loss of weight, loss of appetite, consumption of oral contraceptives or family history of any malignancy. Abdomen examination showed multiple intra-abdominal mobile non-tender masses of varying size in the pelvis, right hypochondriumand right lumbar regions with the mass in pelvis being the largest, measuring approximately 15 x 8 cm. Rectal examination revealed extraluminal mass anteriorly at 5 cm from anal verge which was confirmed during per vaginal examination as well. There was no ascites.
Haematological investigations were normal. Ultrasound abdomen revealed a solid mass in pelvis and another mass in the right subhepatic region with no free fluid. Contrast-enhanced computed tomography (CECT)of the abdomen revealed multiple intraperitoneal masses, pelvic lobulated mass (20x13 cm) displacing the rectum posteriorly and uterus anteriorly, right subphrenic mass (11x19 cm) and multiple small other peritoneal masses in the right iliac fossa of varying sizes.The liver and pancreas and the rest of solid organs were normal, with no gross thickening in colon or rectum, and no ascites. Considering the possibility of metastatic peritoneal deposits from unknown primary, metastatic work-up was considered.Tumor markers (CA 125, AFP, CA19-9, Beta HCG)were within normal limits.Positron emission tomography revealed multiple non-FDG avid masses corresponding to the findings of CECT abdomen. Given these findings, an ultrasound-guided biopsy was performed which to our surprise revealed features of leiomyoma.
As the patient was symptomatic andthe biopsywas suggestive of leiomyoma, surgical excision was planned. She underwent diagnostic laparoscopy followed by exploratory laparotomy. Operative findings showed normal liver, and no ascites. Multiple well-encapsulated masses in the pelvis, right subhepatic region (Figure 1) and on small bowel mesenteric surfaces were noted, with no direct invasion to it or adjacent organs. The uterus was normal. En-bloc removal of all gross masses was performed uneventfully. A total of 17 tumours were removed. (Figure 2)
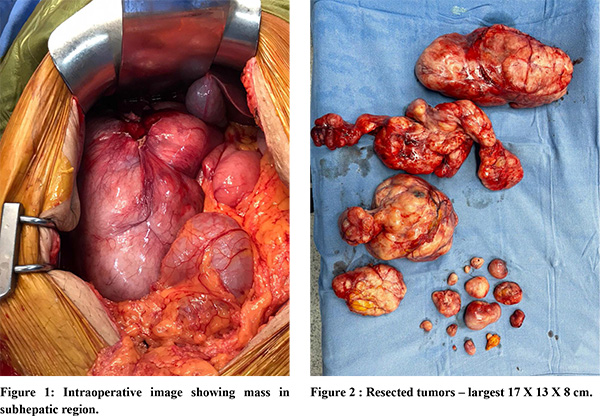
The post-operative period was uneventful and the patient was discharged on day 5. Histopathologic examination revealed neoplastic smooth muscle cells arranged in an intersecting pattern (Figure 3) with focal hyaline change without mitosis or necrosis suggestive of leiomyoma. Immunohistochemistry was positive for Desmin (Figure 4) and negative for SMA (Figure 5), CD34, CD117, DOG-1. Ki 67 was 1-20% which was consistent with the diagnosis of leiomyoma.
Combining the morphological and histological features a final diagnosis of leiomyomatosis peritonealis disseminata was made.
At 1 year of follow up patient is doing well and asymptomatic with no recurrence.
Discussion
Leiomyomatosis peritonealis disseminata (LPD) are rare reported benign tumours arising from sub-mesothelial tissues of abdominopelvic organs. Described first by Wilson et al in 19523 these tumours are scattered all around the peritoneal surface and mimic metastatic lesions from ovaries or lymphomas. The diagnosis is established based on the morphology and histological evidence of bundles of smooth muscle cells with no necrosis or atypia. They are mostly asymptomatic until they attain significant size to cause obstructive bowel symptoms or may present as pain abdomen as was seen in our case. Cross-sectional imaging shows multiple circumscribed lesions without any hallmark radiological characteristics. PETCT scan may help in differentiating a benign from a malignant lesion. However it still cannot be considered a diagnostic modality with accuracy. The diagnosis is confirmed with histology and IHC showing interlacing bundles of smooth muscle cell proliferation, not associated with nuclear atypia or mitotic figure and absence of necrosis.Immunohistochemistry is necessary for confirmation, which usually shows typical smooth muscle pattern. Other leiomyomas like IVL, BML, retroperitoneal, parasitic myoma, or diffuse leiomyomatosis of the uterus may also have similar histopathology but intraoperative findings may help to rule them out.
LPD is usually seen in females of reproductive age group. Very rarely they have been reported in post-menopausal females and males2. Various risk factors associated with LPD are pregnancy, long-term contraceptive use, and previous surgery, especially laparoscopic uterine myomectomy1. The exact etiopathogenesis of LPD is unknown but it is speculated that implantation and dissemination of uterine fibroid tissue during myomectomy or during specimen retrieval by morcellation after laparoscopic uterine surgeries may contribute to it. Our case had a history of laparoscopic myomectomy performed 7 years back which could have been the reason for implantation of myoma tissue into the peritoneum leading to the current scenario. Another proposed hypothesis is metaplasia and differentiation of mesenchymal stem cells into smooth muscle cells under the influence of estrogen leading to LPD4.
The rare presence of LPD in males is not explainable by these hypotheses,which also hint towards the possible role of genetics in the development of this disorder.
No guidelines exist for the management of LPD due to paucity of data. Treatment should be individualised taking into consideration the patient’s age, desire for conception and symptomatology. Surgical excision is the treatment of choice in patients with large tumours who are symptomatic. In patients who have completed their family, it can be combined with hysterectomy and bilateral salpingo-oophorectomy. Alternatively, GnRh (gonadotropin-releasing hormone) analogue therapy should be considered2.
Recurrence after surgical removal of LPD is known5. Gonadotropin-releasing hormone, aromatase inhibitor or selective progesterone receptor modulator can be used to prevent recurrences. Malignant transformation though rare, has been reported in 2-5% cases. Hence, it is advisable for close surveillance after surgical removal using imaging modalities like vaginal ultrasound or magnetic resonance imaging.
Conclusion
LPD is a rare benign disorder, most commonly present in reproductive-age females. Presentation and imaging findings of LPD are non-specific. Diagnosis of these lesions requires a high degree of suspicion andis often misdiagnosed as peritoneal carcinomatosis/lymphoma. Definite diagnosisis made only by histopathology. Surgical excision is the treatment of choice in large symptomatic lesions.
References
- Marwah N, Duhan A, Aggarwal G, Sen R. An unusual presentation of pelvic leiomyomatosis misdiagnosed as disseminated malignancy. Case Rep Pathol. 2012;2012:394106.
- Soni S, Pareek P, Narayan S. Disseminated peritoneal leiomyomatosis: an unusual presentation of intra-abdominal lesion mimicking disseminated malignancy. Med Pharm Rep. 2020 Jan;93(1):113–6.
- Willson JR, Peale AR. Multiple peritoneal leiomyomas associated with a granulosa-cell tumor of the ovary. Am J Obstet Gynecol. 1952 Jul;64(1):204–8.
- Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008;28(7):1931–48.
- Li J, Dai S. Leiomyomatosis Peritonealis Disseminata: A Clinical Analysis of 13 Cases and Literature Review. Int J Surg Pathol. 2020 Apr;28(2):163–8.