|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
Irritable Bowel Syndrome, Fecal Microbiota Transplant, Colonoscopic FMT. |
|
|
|
Avnish Kumar Seth1, Kritika Saxena2, Samir Parikh2 1Department of Gastroenterology & Hepatobiliary Sciences, 2Department Mental Health & Behavioural Sciences, Fortis Memorial Research Institute, Sector 44, Gurugram, Haryana, India.
Corresponding Author:
Dr Avnish Kumar Seth Email: akseth2003@yahoo.com
DOI:
http://dx.doi.org/10.7869/tg.651
Abstract
Background and Aim: To study effect of FMT in patients with moderate or severe IBS. Methods: Patients with IBS for more than one year were offered three sessions of colonoscopicFMT in addition to standard of care. Severity of disease, psychological distress and QOL were assessed by IBS-SSS, HAM-D, HAM-A and WHO-QOL scores. Results: Ten patients with IBS (IBS-D 5, IBS-C 4 and IBS-M 1) were studied. Median IBS-SSS reduced from 313.5 (SD ± 66.8) at baseline to 163 (SD ± 84.5) at 1 week (p = 0.0005), 216 (SD ± 79.3) at 2 weeks (p = 0.003), 201(SD ± 86.6) at 4 weeks (p = 0.005) and 262 (SD ± 69.4) at 8 weeks. Median IBS-SSS at 12 weeks and 24 weeks was not significantly different from baseline. Reduction of IBS-SSS severity was seen in 8 (80%) patients at one week, 6(60%) at 2 and 4 weeks, 3(30%) at 8 weeks and 1(10%) at 12 and 24 weeks. weeks. Of four patients with depression, there was improvement in two patients at 2 and 4 weeks and one at 8 weeks. Quality of life improved in four patients at 2, 4 and 8 weeks, two patients at 12 weeks and one at 24 weeks. Three patients reported marked improvement of symptoms at 12 months along with change in stool odor to donor type. Conclusion: FMT results in short-term improvement in global symptoms of IBS, psychological distress and QOL. Repeat sessions of FMT did not accrue additional benefit.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|3039 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Of all the functional gastrointestinal disorders, Irritable Bowel Syndrome (IBS) is the most common with a worldwide prevalence of 12%1. Defined by Rome-IV criteria, IBS is classified into four subtypes: IBS with predominant constipation (IBS-C), predominant diarrhea (IBS-D), mixed bowel habits (IBS-M) or unsubtyped(IBS-U)2. The pathophysiology of IBS is multifactorial with influence of infections, diet, gut microbiome and a complex brain-gut interaction, thus presenting considerable difficulty in treatment. Fecal Microbiota Transplantation (FMT) is the term used to describe the infusion of a fecal suspension from a healthy individual into the gastrointestinal tract of another individual. FMT is now an established indication for refractory Clostridium difficile infection3. A recent meta-analysis that included four randomized control trials concluded that FMT may be more effective than placebo in inducing clinical remission and clinical response in patients with Ulcerative Colitis4. We carried out the first successful FMT for Ulcerative Colitis in India in 20145. Role of FMT in disorders like Irritable Bowel Syndrome (IBS), Crohn’s disease, Alcoholic liver disease, Parkinson’s, Autism, Metabolic syndrome and Idiopathic Thrombocytopenic Purpura is under investigation6. Up to 30% of patients with IBS are post-infectious and dysbiosis is now accepted as a predominant factor in the pathophysiology of IBS with relative abundance of proinflammatory bacteria like Enterobacteriaceae and a reduction in Lactobacillus and Bifidobacterium7. New strategies of treatment of IBS are focusing on gut bacteria modulation, of which FMT features prominently8. We carried out an open label single arm cohort study on FMT in patients with moderate or severe IBS.
Methods
Patients with IBS of more than one-year duration, who remained symptomatic despite medical therapy, were offered FMT in addition to standard of care. The diagnosis of IBS was based on Rome IV criteria and sub-classification of IBS was based on the Bristol stool type9. The severity of disease was assessed by IBS-Symptom Severity Score (IBS-SSS), where a score of 75 to 174 indicated mild symptoms, 175 to 299 moderate IBS and score of 300 and above severe IBS10. Pain abdomen was considered to be significant if the pain score was above 50 in the IBS-SSS. The associated psychological distress and quality of life were be assessed by Hamilton Rating Scale for Depression (HAM-D), Hamilton Rating Scale for Anxiety (HAM-A) and World Health Organization Quality of Life (WHO-QOL) scales11,12,13. HAM-Dscore of 8 to 13 was indicative of mild depression, 14 to 18 moderate, 19 to 22 severe and 23 or higher,very severe depression. HAM-A score of 17 or less was suggestive of mild anxiety, 18 to 24 moderate and 25 to 30 severe symptoms. Patients with pregnancy, severe anemia, active gastrointestinal infection, antibiotic or probiotic use over last 30 days, chronic kidney disease, cirrhosis of liver, cardiac failure, malignancy, uncontrolled diabetes mellitus, and uncontrolled thyroid disorderswere excluded. Recipient evaluation included exclusion of other diseases with complete blood count, C-reactive protein, serum Chromogranin A & B, upper gastrointestinal endoscopy and duodenal biopsy, colonoscopy with random biopsy, contrast enhanced CT scan of abdomen, Hydrogen breath tests for lactose intolerance and Small Intestinal Bacterial Overgrowth (SIBO). Also, stool was tested for ova and cysts, culture, Calprotectin, modified Ziehl Nielson stain, Cryptosporidium antigen and Clostridium difficile toxin A and B assay. Donor screening and testing was carried out as per European consensus on FMT in clinical practice to rule out communicable ?diseases, gastrointestinal, rheumatic, allergic and metabolic disorders14. Stool donors who received antibiotics within the preceding 3 months were excluded. 200 gram of donor stool, collected with-in 6 hours of FMT, was blended, filtered and drawn up into a total of 7 syringes of 50 ml each and instilled following informed consent, into the recipient colon at colonoscopy. Thereafter, 2 sessions of FMT were performed fortnightly with instillation of 50 gram of stool from same donor in 50 ml of water into the left colonat flexible sigmoidoscopy. The IBS-SSS was assessed at 1, 2, 4, 8,12 and 24 weeks and HAM-D, HAM-A and WHO-QOL were assessed at 2, 4, 8, 12 and 24 weeks following FMT. The pre-intervention medication remained unchanged for six months after FMT, following which lowest dose was taken to maintain remission. A follow-up questionnaire was used at one year for assessment of symptoms, requirement of medication and to determine the over-all experience of patients following FMT.
Outcome measures
The primary outcome studied was improvement in global symptoms of IBS as defined by reduction in severity of IBS-SSS from severe to moderate/mild or moderate to mild. Secondary outcomes included improvement in HAM-D and HAM-A and WHO-QOL. The parameters of responders and non-responders were compared in an attempt to identify factors that determined response. Relapse was defined as return of IBS-SSS to the pre-intervention severity in patients with response. Adverse events were monitored prospectively.
Statistical methods
Paired T Test was used to compare mean and standard deviation for IBS-SSS scores before and after FMT. Each entity was measured twice, resulting in pairs of observations as one tail and two tail. The age and duration of IBS among responders and non-responders was compared with Mann-Whitney U test. The Fisher Exact test was used to analyze the association of SIBO, lactose intolerance, anxiety and depression between responders and non-responders.
Results
Ten patients, median age 34 (range 18 to 70) years, including 8 males, were studied. The median duration of IBS was 10 (range 5 to 30) years, with five classified as IBS-D, four as IBS-C and one IBS-M. Significant pain abdomen with scores of above 50 in IBS-SSS was present in 5(50%). Three patients had associated lactose intolerance, one had SIBO and two had both lactose intolerance and SIBO (Table 1). Baseline median IBS-SSS was 360 (range 260 to 390) with moderate IBS in five and severe IBS in five patients. Six patients had depression at baseline with median HAM-D score of 8(range 0 17). Two patients had high HAM-A scores of 19 and 22 and both had mixed anxiety depression disorder. The median WHO-QOL overall score at baseline was 59 (range 44 to 87). The median age of stool donors was 33 years (range 25 to 72 years) with six males and three spousal donors. All patients completed the FMT protocol.
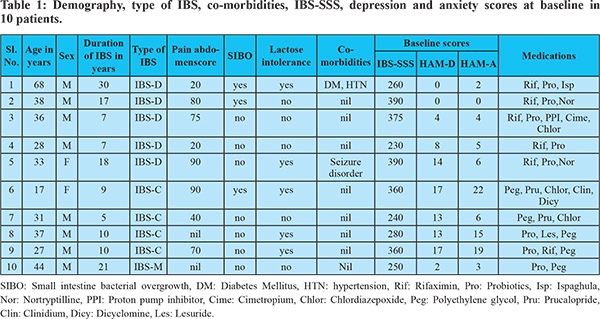
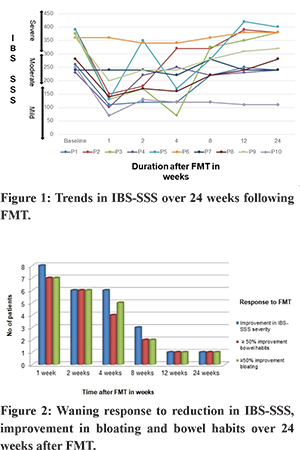
The trends of IBS-SSS over 24 weeks following FMT in the 10 patients are shown in Figure 1. Median IBS-SSS significantly improved at week 1, 2, 4, and 8 but not at week 12 and 24. Median IBS-SSS reduced from 313.5 (SD ± 66.8) at baseline to 163 (SD ± 84.5) at 1 week (p = 0.0005), 216 (SD ± 79.3) at 2 weeks (p = 0.003), 201(SD ± 86.6) at 4 weeks (p = 0.005) and 262 (SD ± 69.4) at 8 weeks. Median IBS-SSS at 12 weeks, 292 (SD ± 95), and 24 weeks, 294 (SD ± 95.1), was not significantly different from baseline (p = 0.19 and 0.21, respectively). Reduction of IBS-SSS by at least one grade was noted in 8 of 10 (80%) patients at one week after FMT, 6 of 10(60%) at 2 and 4 weeks, 3(30%) at 8 weeks and 1(10%) at 12 and 24 weeks. The temporal profile of response to IBS-SSS and correlation with more than 50% improvement in bloating and bowel habits is shown in Figure 2. Of the five patients who had IBS-SSS pain score of more than 50 at baseline, greater than 50% reduction in pain abdomen was seen in four patients at 1,2 and 4 weeks, two at 8 weeks and none at 12 and 24 weeks.
Age of patients, duration of IBS, type and severity of IBS, associated SIBO or lactose intolerance and prevalence of depression or anxiety did not predict response at four weeks (Table 2). Repeat sessions of FMT by sigmoidoscopic instillation at two and four weeks had a positive impact on IBS-SSS in only one patient out of ten.
Of four patients with depression, there was improvement in grade of HAM-D score in two patients at 2 and 4 weeks, one patient at 8 weeks and none of the patients at 12 and 24 weeks. One of the two patients with mixed anxiety depression disorder showed improvement at 2 and 4 weeks only. All patients who had improvement in IBS-SSS showed improvement in HAM-D and HAM-A. Quality of life improved in four (40%) patients at 2, 4 and 8 weeks and two patients at 12 weeks and one at 24 weeks. Three of these four patients had shown clinical response to IBS-SSS while one showed improvement in WHO-QOL despite clinical non-response. None of our patients reported significant adverse events following FMT. Two (20%) patients developed transient abdominal cramps and vomiting for 12 hours following FMT. One patient reported shifting in site of pain from left lower abdomen to central abdomen three months following FMT. In response to a questionnaire at the end of one year, seven out of ten patients did not regret enrolling for the trial on FMT and said that they would not mind doing it again provided there was a better alternative to processing stool at home. Six of them said they would recommend FMT to others based on their own experience. Five patients reported change in odor of their stool to donor stool following FMT that lasted for about two months. Interestingly, three patients, all of whom had relapse following initial improvement, reported marked improvement of symptoms at 12 months. They reported that the odor of their stool changed to that of the donor at about nine to ten months post FMT with more than 50% reduction in their symptoms with two of them being off all medication.
Discussion
The results of our study, the first of its kind from India, are very similar to the few other open label studies on colonoscopic FMT for IBS. Cruz et al. from Germany reported transient improvement in nine patients with profound changes in the microbiome of patients with IBS-D15. Hong et al. from Korea reported improvement in IBS-SSS in 80% patients at one month in 12 patients, but the symptoms returned to baseline at 3 months16. In a meta-analysis of single arm trials by Myneedu et al, 59.5% (95% confidence interval (CI) 49.1–69.3) of IBS patients showed significant improvement at 12 weeks. However, in their meta-analysis of 5 RCTs, there was no significant difference between FMT and controls in clinical improvement at 12 weeks (RR1/40.93 (95% CI 0.50–1.75) or in quality of life17. The results of six RCTs on FMT for IBS are summarized in Table 3. Despite the immense variability in study design and delivery of FMT in these studies, there are 3 systematic reviews and meta-analysis in patients with IBS. Xu et al. showed no significant difference in global improvement of IBS symptoms at 12 weeks in FMT vs placebo (RR 5 0.93; 95% CI 0.48–1.79). Two RCTs that used FMT through colonoscopy and nasojejunal tube demonstrated a clinically significant improvement in global IBS symptoms. On the contrary, the two RCTs with use of oral capsule for delivery of FMT showed no benefit or even worse outcome in one of them24. Another meta-analysis by Ioniro et al. also concluded that fresh or frozen donor stool delivered via colonoscopy or nasojejunal tube may be beneficial in IBS. Delivery of FMT by oral capsules was not beneficial25. The reasons for these differences may be explained by variation in route of administration and placebo effect. There is evidence to suggest that bowel lavage and colonoscopy by themselvescan alter the fecal microbiota favorably for a few weeks26. The response to FMT in our patients with IBS was transient and waned after 8 weeks in the majority of patients. The improvement in symptoms was most apparent at one week after FMT. Some patients felt ‘like never before’ and even sent thank you notes and texts. It has been shown that there is incremental effect of donor stool on gut flora of the recipient that may be demonstrable as early one week after FMT but it may take three months for the biodiversity to match that of the donor27. If FMT was indeed effective for IBS, the improvement should have been most obvious at 12 weeks and even continued beyond. It is quite likely that the transient response in our patients was due to placebo effect. In a meta-analysis of 73 RCTs on treatment of IBS, pooled placebo response was as high as 37.5% (95% CI 34.4-40.6%)28. Some experts feel that the delivery of fecal bacteria to the upper GI tract may inadvertently cause an exacerbation of underlying functional GI symptoms. However, the RCT by El-Salhy et al, published after all three meta-analyses, showed that endoscopic delivery of FMT resulted in response at 3 months in 23.6%, 76.9% (p<0.0001) and 89.1% (p<00.0001) of the patients who received placebo, 30 g FMT and 60 g FMT23.
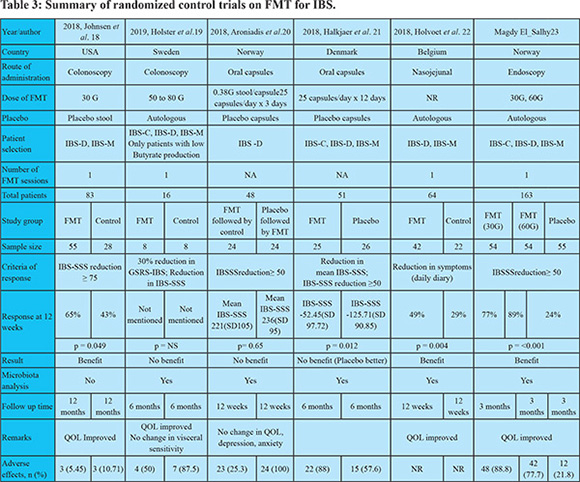
It is interesting to note that the criteria of response in the six the RCTs is quite variable. When we analyzed our data based on downgrading of severity of IBS-SSS from severe to moderate/mild or from moderate to mild, reduction in IBS-SSS by 75 or more or a reduction in IBS-SSS by 50 or more, the outcomes remained similar (Figure 3). The European Medicines Agency and the US Food and Drug Administration suggest the use of reduction in abdominal pain as the primary endpoint and assessing stool frequency as a secondary endpoint in studies on IBS. However, as suggested by the National Task Force on IBS in India, pain abdomen may not be ubiquitous in patients with IBS in our country29. Type of IBS did not influence the result of FMT in our study. Majority of patients included in the six RCTs had IBS-D or IBS-M, and very few had IBS-C. It is, however, also well known that patients classified by the same IBS subtype do not necessarily display the same pathophysiology.
Changes in gut microbiota in patients with IBS have been well described. A meta-analysis performed on 13 studies had shown that there were significant differences in expression in IBS patients compared to healthy controls for Lactobacillus, Bifidobacterium, and Faecalibacteriumprausnitzii30. The gut microbiota profile of the donor should ideally be able to reinforce the deficient species in patients with IBS. However, as demonstrated by some of the RCTs, favorable changes in gut microbiota may not necessarily correlate with clinical response to FMT19,20,21,22,23. Improvement in WHO-QOL, like improvement in IBS-SSS severity, was transient in our patients. One patient had improvement in WHO-QOL despite no improvement IBS-SSS. In a study of 161 patients followed up for 4.7 years, reduction in symptoms of IBS did not correlate with improvement in QOL. Also, higher anxiety and depression scores at follow-up were associated with lower QOL and life satisfaction at follow-up31. In a recent double-blind randomized placebo-controlled study on patients with non-constipated IBS, significant improvement in QOL (Odds ratio (OR) 3,801; confidence interval (CI) = 1,309 11,042 p = 0.011) and fatigue (OR = 4,398; CI = 1,175 16,468 and p = 0,020) was found at six months(32). Adverse events were limited to mild and transient gastrointestinal symptoms in our patients.The adverse effects reported in treatment and placebo arms of all six RCTs are shown in Table 2. Response to our questionnaire at one year threw up some information on acceptance of FMT by patients of IBS and willingness to undergo the procedure again. The recipients could discern a change in the odor of their stool following the procedure. The improvement in three of our patients at nine months with return of donor-type stool odor was an observation that may point to a second peak in engraftment of donor microbiota, an aspect that has not been studied. Marked improvement in one of the patients one year following FMT has been reported in one of the RCTs18. FMT resulted in significant benefit in symptoms of bloating, frequency of stool and pain abdomen for eight weeks in our study. Symptoms returned to baseline at 12 weeks in the majority. Improvement in depression, and QOL followed the same pattern. Delayed improvement was noted at 9 months after FMT in some patients.Even though our study is limited by open label design, small cohort of patients and absence of data on effect on bacterial flora, we have shown for the first time, that repeated sessions of FMT may not accrue any additional benefit in patients with IBS. Future trials could consider tailoring the use of FMT to various subtypes of IBS by using of donors with a specific gut microbiome profile.
References - Lacy BE, Patel NK. Rome criteria and diagnostic approach to Irritable Bowel Syndrome. J Clinical Med 2017;6(99);1-8.
- Drossman DA, Hasler WL. Rome IV Functional GI disorders: disorders of gut-brain interaction. Gastroenterol 2016;150:1257-61.
- McDonald LC, Gerding DN, Johnson S et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America. Clin Inf Dis 2018;66(7):e1-e48.
- Lam WC, Zhao C, Ma WJ et al. The clinical and steroid-free remission of Fecal Microbiota Transplantation to patients with ulcerative colitis: a meta-analysis. Gastro Res and Practice 2019(8630): 1-10.
- Seth AK, Rawal P, Bagga R et al.. Successful stool transplantation for severe ulcerative colitis: First report from India. Indian J Gastroenterol 2016;35(5):393-5. DOI 10.1007/s12664-016-0696-2.
- Lee WJ, Lattimer LDN, Stephen S et al. Fecal MIcrobiotaTransplantattion: a review of emerging indications beyond relapsing Clostridium difficile toxin colitis. J Gastro Hepat 2015; 11(1): 24-32.
- Rodino-Janeiro BK, Vicario M, Alonso-Cotoner C et al. A review of microbiota and Irritable Bowel Syndrome: Future in therapies. Adv Ther 2018. https://doi.org/10.1007/s12325-018-0673-5
- Harris L A, Baffy N. Modulation of the gut microbiota: a focus on treatments for irritable bowel syndrome. Postgrad Med 2017; 129(8);872-888.
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol1997, 32, 920–924.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment PharmacolTher. 1997 Apr;11(2):395-402.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62.
- Hamilton M.The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55.
- The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): Development and general psychometric properties. Soc Sci Med. 1998;46:1569–1585.
- Cammaroto G, Laniro G, Tilg H et al. European consensus conference on fecal microbiota transplantation in clinical practice. Gut 2017; 0:1-12. doi:10.1136/gutjnl-2016-313017.
- Cruz-Aguilar R, Buch T, Bajbouj M, et al. Fecal micro- biota transplantation as a novel therapy for irritable bowel syndrome with predominant diarrhea. NeurogastroenterolMotil 2015; 27: 110.
- Hong J, Bang B, Shin Y, et al. Treatment of irritable bowel syndrome with fecal microbiota transplantation: a case series of 10 patients. United European Gastroenterol J 2016(Suppl 2):2.
- Myneedu K, AbhizithDeoker A, Schmulson MJ, Bashashati M. Fecal microbiota transplantation in irritable bowel syndrome review and meta-analysis. United European Gastroenterology Journal 2019;7(8): 1033-1041. https://doi.org/10.1177/2050640619866990.
- Johnsen, PH, Hilpusch, F, Cavanagh, JP, et al. Faecal microbiota transplantation versus placebo fo severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, sin Lancet Gastroenterol Hepatol 2018; 3: 17–24.??
- Holster S, Lindqvist CM, RJ, Repsilber, D, et al. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clinical and Translational Gastroenterology 2019;10:e-00034. https://doi.org/10.14309/ctg.0000000000000034.
- Aroniadis, OC, Brandt, LJ, Oneto, C, et al. A double-blind, randomized, placebo-controlled trial of fecal microbiota transplantation capsules (FMTC) for the treatment of diarrhea-predominant irritable bowel syndrome. Lancet Gastroenterol Hepatol 2019, published online July 17, 2019. http://dx.doi.org/10.1016/S2468-1253(19)30198-0.
- Halkjaer, SI, Christensen, AH, Lo, BZS, et al. Faecal microbiota transplantation alters gut microbiot irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 2018;0:1-9. ? doi:10.1136/gutjnl-2018-316434.
- Holvoet, T, Joossens, M, Jerina, B, et al. Fecal microbiota transplantation in irritable bowel syndrom abdominal bloating: results from a double blind, placebo-controlled clinical trial. Gastroenterology 2 S130–S130.?
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut2020;69:859–867.
- Xu D, Chen VL, Steiner CA, et al. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019 Jul;114(7):1043-1050. doi:10.14309/ajg.0000000000000198.
- Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of fecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharma Therap 2019; 50(3): 240-8. https://doi.org/10.1111/apt.15330.
- Drago L, Toscano M, De Grandi R, Casini V, Pace F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol. 2016; 28: 532– 537.
- Garza-Gonzalez E, Mendoza-Olazaran S, Morfin-Otero R, et al. Intestinal microbiome changes in FMT Vs FMT enriched with Lactobacillus in the treatment of recurrent Clostridioides difficile infection. Canadian J Gastroenterol 2019, Article ID 4549298, 7 pages. https://doi.org/10.1155/2019/4549298.
- Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in irritable bowel syndrome. Aliment Pharma Ther 2010;32:144-158.
- Ghoshal UC, Abraham P, Bhatt C et al. Epidemiology and clinical profile of Irritable Bowel Syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol 2008;27:22-28.
- Liu et al., 2017.Chong PP, Chin VK, Chung YengLooi, Wong WF, Madhava P, Yong VC. The Microbiome and Irritable Bowel Syndrome – A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol 2019; 10: 01136.
- Weerts ZZRM,Vork L, Mujagic Z, et al. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol and Motility 2019;00:e13629.
- Johnsen PH, Hilpusch F, Valle PC, Rasmus Goll R. The effect of fecal microbiota transplantation on IBS related quality of life and fatigue in moderate to severe non-constipated irritable bowel: Secondary endpoints of a double blind, randomized, placebo-controlled trial. Ebiomed 2020 (51) 102562. https://doi.org/10.1016/j.ebiom.2019.11.023.
|
|
|
 |
|
|