|
Ramesh Kumar1, Utpal Anand2, Rajeev Nayan Priyadarshi3 1Department of Gastroenterology, All India Institute of Medical Sciences, Patna, India. 2Department of surgical gastroenterology, All India Institute of Medical Sciences, Patna, India. 3Department of Radiodiagnosis, All India Institute of Medical Sciences, Patna, India.
Corresponding Author:
Dr Ramesh Kumar Email: docrameshkr@gmail.com
DOI:
http://dx.doi.org/10.7869/tg.592
Abstract
The current definition of Chronic kidney disease (CKD) in patients with liver cirrhosis is based on decline in glomerular filtration rate (GFR)<60ml/min per 1.73m2 for 3 months. It includes both the organic-CKD due to structural damage to kidney; and functional-CKD due to circulatory and neurohormonal imbalances in cirrhosis. Emerging data suggest that incidence of CKD has considerably increased in cirrhotic patients over last decade. The available data on this condition is extremely limited. Many issues related to diagnosis and management of CKD in cirrhosis lack clarity which pose several challenges to the treating clinicians. The assessment of renal dysfunction and its chronicity is difficult in cirrhotic patients due to variable overestimation of GFR measured by creatinine-based formula, absence of reliable biomarkers of CKD and difficulty in performing renal biopsy in advanced cirrhotic patients. With both liver and kidney being dysfunctional, fluid mobilization to control ascites and edema becomes problematic. The diuretic resistance or contraindication are common, requirement of therapeutic paracentesis is often very high, transjugular intrahepatic portosystemic shunt is associated with very high risk of hepatic encephalopathy and hemodialysis is poorly tolerated in such patients. Moreover, prescribing medicines in patients with concomitant hepatorenal dysfunction is a challenging task. It is difficult to predict the reversibility of functional-CKD following liver transplantation, which complicates the decision regarding the need of simultaneous liver and kidney transplantation. This article provides a comprehensive review of current concepts, controversies and challenges with regard to the diagnosis, clinical evaluation and management of such patients.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|2978 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Patients of liver cirrhosis are prone to develop renal impairments because of pre-existing circulatory disturbances, neurohormonal imbalances and presence of risk factors such as diabetes mellitus (DM), bacterial infection, gastrointestinal bleeding, drugs, therapeutic paracentesis, etc.1-4. Various types of renal impairments have been described in this setting, such as acute kidney injury (AKI), chronic kidney disease (CKD) and AKI on CKD3,4. The differentiation between them is imperative as each requires a different treatment strategy. So far, the most efforts on evaluating renal impairment in cirrhosis have been concentrated on AKI, and substantial information is now available on AKI in cirrhosis. However, the data on prevalence, clinical impact and management of CKD in cirrhosis is still scanty. There appears to be a complex interplay between AKI and CKD. AKI survivors remain at elevated risk of developing de-novo CKD. Depending upon the severity, duration, and frequency, AKI increase the risk of developing incident CKD due to reductions in renal mass and nephron number, vascular insufficiency, and maladaptive repair mechanisms5. Therefore, AKI and CKD may represent a continuum rather than separate entities. With the current definition of CKD based on glomerular filtration rate (GFR) only, the term CKD now includes both the organic-CKD due to structural damage to kidney; and functional-CKD due to circulatory and neurohormonal imbalances in cirrhosis3.
Defining of CKD in Cirrhosis
In 2002, the National Kidney Foundation- Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) proposed the definition and classification for CKD which was subsequently endorsed by the Kidney Disease: Improving Global Outcomes (KDIGO) in 2004. The definition of CKD was based on the presence of kidney damage (urinary albumin-to creatinine ratio >30mg/g) or low GFR (<60ml/min per 1.73/m2) for 3 months, irrespective of cause6. For patients with cirrhosis, the definition of CKD was based on a serum creatinine threshold of =1.5 mg/dL till 2011, when a revised definition was proposed by working party consisting of specialists from multiple disciplines3. This group largely adopted the definition set out by KDIGO and defined CKD as an estimated-GFR (e-GFR) of<60 ml/ min for more than 3 months calculated using the Modi?cation of Diet in Renal Disease 6 (MDRD 6) formula. The group further recognized that the MDRD6 formula was not perfect for the cirrhotic patients and this might change as better alternatives become available. The MDRD6 formula is calculated with the following 6 variables: serum creatinine, age, sex, albumin, blood urea nitrogen, and race. The corroborating evidence of kidney damage such as proteinuria, hematuria, abnormal renal imaging or pathology was not taken into consideration by this group.
Magnitude of CKD in Cirrhosis
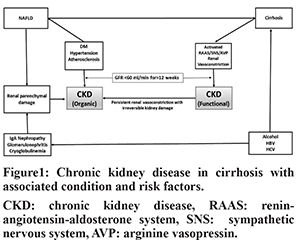
CKD includes both the organic-CKD and functional-CKD. The functional-CKD largely represents type-2 hepatorenal syndrome (HRS) which is now known as HRS-CKD4. Though the functional-CKD is considered a potentially reversible, it may not be truly functional as injury as studies assessing biomarkers of tubular damage have revealed evidence of tubular injury in patients with HRS7-9. A persistent functional-CKD may go on to develop organic CKD. Moreover, patients with cirrhosis may have various risk factorsfor developing organic CKD, such as DM, non-alcoholic fatty liver disease (NAFLD), atherosclerosis, etc. There has been a substantial increase in prevalence of CKD in general population during recent times. The estimated CKD prevalence in general populations varies between 11 to 13%10. The reported prevalence of CKD in hospitalized cirrhotic patients has increased from 1% in 2005 to as high as 46.8% in 201911-18 (Table 1). The inclusion of function-CKD in the diagnostic criteria, growing awareness about this condition and a rising trend in the prevalence of DM, hypertension, and NAFLD appear to be the main reasons behind increased prevalence of CKD in cirrhosis. A study that analyzed prospectively maintained North American Consortium for the Study of End-Stage Liver Disease (NACSELD) database reported 46.8% prevalence of CKD among 2346 admitted cirrhotic patients11. In a recent study from India, 32.8% of 818 of patients with cirrhosis were found to develop CKD during follow-up12. In that study, approximately 80% of patients had at least one episode of AKI, and one third of patients with AKI were found to progress to CKD. The functional-CKD is a relatively uncommon and accounts for 3.9% to 15.8% of renal impairments among hospitalized cirrhotic patients17,18. DM and hypertension account for over two-third of the cases of CKD in general population. The reported prevalence of diabetes in cirrhosis varies from 35% to 71% which is much higher than that in general population19. The prevalence of NAFLD-related cirrhosis has increased considerably over the last 2 decades. NAFLD is significantly associated with increased incidence and prevalence of CKD20,21. NAFLD can accelerate the development and progression of CKD independent of traditional risk factors. A recent meta-analysis that included nearly 64,000 subjects found that NAFLD was associated with an approximately 2-fold increase risk of both prevalent and incident CKD21. The pro-inflammatory milieu along with insulin resistance, dyslipidemia, oxidative stress, hypertension, activated renin-angiotensin system may account for accelerated development and progression of CKD in NAFLD subjects22. In a study by Singal et al, NAFLD accounted for a significant increase in simultaneous liver and kidney (SLK) transplantation from 8.2% in 2002 to 22% in 201123. Certain specific cause of cirrhosis may be associated with glomerulopathy which may progress to CKD (Figure 1). Also, the prevalence of atherosclerosis, which has association with glomerulosclerosis, is not uncommon in cirrhosis as indicated by fact that around 20-25% of candidates for liver transplantation (LT) have significant coronary artery disease24.
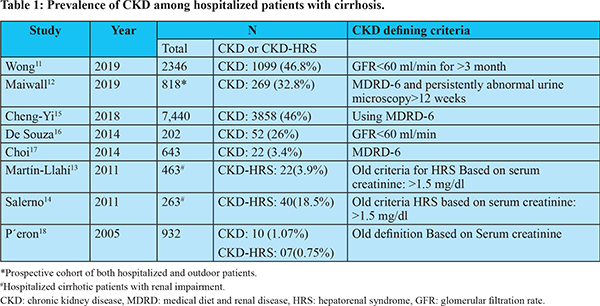
A sharp increase in the diagnosis of CKD in cirrhotic patients calls into question whether relying only on eGFR in the absence of any corroborating evidence of kidney damage leads to overdiagnosis of CKD25. An arbitrary single threshold of eGFR (<60 mL/min/1.73 m2) has high propensity to cause overestimation of CKD in the elderly subjects. There is a normal steady decline in GFR with increasing age, and individuals >55 years of age with eGFR of <60 ml/min may have very little tendency to progress to symptomatic kidney disease, but still, they will be labelled as having CKD26. Because many cirrhotic patients belong to this age group, the chances of overdiagnosis of CKD in them would be substantial. Moreover, a varying degree of decline in GFR can occur in patients with decompensated cirrhosis because of neurohormonal alterations and circulatory dysfunction, even before onset of HRS, which may further contribute to overdiagnosis of CKD, particularly in elderly cirrhotic. Therefore, clinicians need to be skeptical about the labelling CKD based on single threshold of eGFR, particularly in older cirrhotic (>55 years) and in absence of any corroborating evidence of kidney damage.
Assessment of Renal Function in Cirrhosis
Estimation of GFR
The GFR is currently the best indicator of renal function. The gold standard for measurement of GFR is direct Iothalamate clearance test27. However, cumbersome technique and lack of widespread availability limit its use in clinical practice. Creatinine-based formula is usually used to estimate GFR in clinical practice. However, in cirrhotic patients, serum creatinine level may be unreliable due to: hepatic dysfunctions causing decreased production of endogenous creatine; reduced skeletal muscle mass causing decreased conversion of creatine to creatinine; increased tubular secretion of creatinine; and underestimations of serum creatinine estimation caused by hyperbilirubinemia28-30. Therefore, creatinine-based equation tends to overestimate GFR and a normal serum creatinine level cannot exclude renal impairment in cirrhotic patients29,30. In a meta-analysis, creatinine-based formula overestimated GFR by 18.7 ml/min/1.73 m31. A serum creatinine of >1.5 mg/dL usually corresponds to a GFR of <30 mL/min in cirrhotic patients. Despite limitations, the MDRD is a widely used creatinine-based equation to estimate GFR in cirrhosis. The latest equation MDRD-6 has been recommended by expert panel until better substitutes become available3,28.
Biomarkers of kidney damage
The role of conventional urinary markers such as albuminuria is very limited in patients with cirrhosis, which may be because of hypoalbuminemia and relatively increased capillary permeability28. Several types of parenchymal changes can occur in kidney in the absence of abnormal urine-analysis. Also, a normal quantitative proteinuria does not exclude glomerular changes32. Cystatin C, a protein produced by all nucleated cells in the body and eliminated exclusively by glomerular filtration, is an alternative method for estimating the GFR33-35. It may be better alternative to serum creatinine in cirrhotic patients because it is independent of hepatic function, muscle mass, sex, hyperbilirubinemia and its tubular secretion35. However, measurement of cystatin C is influenced by low serum albumin levels, elevated C-reactive protein levels and leukocytosis which may limit its roleto estimate GFR in cirrhosis37. Several cystatins C-based GFR equations have been described; however, the diagnostic performance of all of them is lesser in cirrhotic patients than in those without cirrhosis28. Also, studies have shown that combined serum creatinine and cystatin C in an equation predicts GFR more accurately than those using either alone36,38. Urinary neutrophil gelatinase-associated lipocalin (NGAL) has been shown to be significantly elevated in CKD patients compared to controls9,39. Its levels increase with stages of CKD suggesting a role in following progression of CKD40. However, role of increased urinary-NGAL in the setting of CKD needs further evaluation. Other biomarkers of tubular injury including osteopontin and tissue inhibitor of metalloproteinases-1 (TIMP-1) are usually increased in CKD patients41; however, their clinical significance is yet to be established. The urinary micro-RNAs profiles may be an attractive non-invasive tool to assess kidney damage in future28.
Differentiation Between Organic and Functional CKD
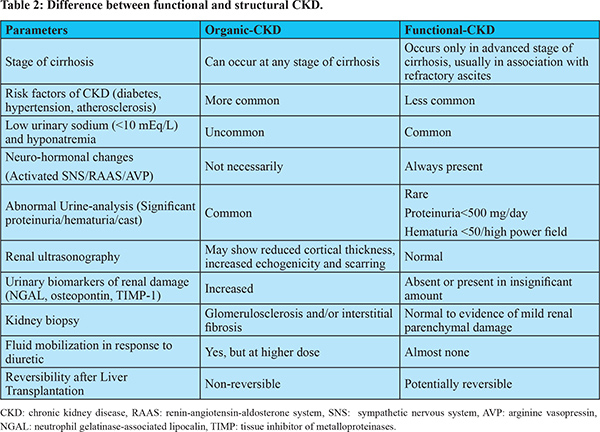
The differentiation between organic and functional CKD has important therapeutic and prognostic implications. Such differentiation would assist clinician in taking decision with regard to the use of diuretics, vasoconstrictors treatment and recommending transplantation of liver only versus SLK. Functional-CKD is potentially reversible after LT, though, it is not yet clear as to how long it remains to be reversible as prolonged kidney ischemia can result in irreversible kidney changes13,14. Studies on outcome of LT in patients with type-2 HRS patients have found that, even if HRS reverses, 50-60% of patients develop stage-3 CKD during the first post-transplant period42,43. It is often difficult to differentiate a functional-CKD from the organic one because of lack of reliable biomarkers capable of detecting subclinical renal parenchymal damage. In absence of abnormal imaging, reliable biomarkers and abnormal urine analysis, kidney biopsy remains the only option to diagnose and further characterize CKD. However, percutaneous renal biopsy may be challenging in patients with decompensated cirrhosis because of coagulopathy and significant thrombocytopenia. Moreover, transjugular kidney biopsy may also be technically difficult in presence of large ascites and scarred kidney44. In clinical practice, functional-CKD should be diagnosed by exclusion. Table 2 depicts some of important differentiating points between functional and organic CKD.
Clinical Course, Complications and Outcome
Both hepatic and renal dysfunction can independently lead to anorexia, anemia, ascites, bleeding tendency and encephalopathy. Because of multiple similarities in clinical manifestations, contribution from individual disease is often difficult to be determined in patients with cirrhosis and CKD. This often creates uncertainty about optimal therapeutic decisions such as requirement of renal replacement therapy. Refractory ascites is almost universal in association with functional-CKD13,14. The evaluation of ascites in cirrhotic patients with CKD needs consideration of various factors other than portal hypertension such as nephrogenic ascites, chronic fluid overload, hypoproteinemia, peritonitis, associated cardiomyopathy, etc.45. Both hepatic and renal dysfunction cause bleeding tendency due to multiple and complex abnormalities of hemostasis. Despite the bleeding tendency, hypercoagulability and thrombotic complications in such patients are not unusual46. Both CKD and cirrhosis can cause immunodepression leading to increased risk of infection47. Advanced CKD can lead to altered body metabolic activities and retention of toxic substances affecting various organ systems of the body. CKD can worsen anemia of cirrhosis48. Also, CKD can lead to various metabolic complications, including hypoalbuminemia, hyperkalemia, hyperparathyroidism, and hyperphosphatemia49. These metabolic complications have strong association with GFR. Hyperuricemia and organic anions in CKD patients interfere with loop diuretics50. CKD is independent risk factor for cardiovascular mortality49. CKD in cirrhosis is associated with complications and poor outcome. In a recent study, Wong et al found that cirrhotic patients with CKD (n=1099) had higher rates of superimposed AKI (68% versus 21%; P < 0.001), need for dialysis (11% versus 2%; P < 0.001), hospitalization in previous 6 months and mortality at 30 days or 90-days than those without CKD11. Another study from Europe has found that cirrhotic patients with CKD have higher risk (26%) of developing superimposed AKI51. Maiwall et al found CKD to be an independent predictor of overall mortality in cirrhosis12. Moreover, presence of cirrhosis is independently associated with poor outcome in patients with CKD52.
Management Issues
The presence of CKD in cirrhotic patients poses several challenges to clinicians with regard to medical management (Table 3). We discuss here various therapeutic options in such patients:
Diuretic Therapy for fluid mobilization
With both liver and kidney being dysfunctional, fluid mobilization to control ascites and edema becomes a real challenge. Diuretic treatment is often not recommended in patients with functional-CKD for the concern that it may further worsen kidney function due to intravascular volume depletion and may aggravate dyselectrolytemia13,14. However, in patients with stable functional-CKD, maintenance of diuretic therapy can be considered if it achieves sizable natriuresis (>30mmol/day) without producing side effects53. In patients with organic-CKD without any evidence of superimposed AKI-HRS, it seems reasonable to use diuretic to control ascites and edema. However, there is paucity of evidence to guide clinicians regarding which diuretic is the ideal in such patients, whether it should be used in combination or when switching from one to another class of diuretic be considered. A varying degree of diuretic resistance is usually present in patients with CKD45. This happens mainly because of decreased renal blood flow, hyperuricemia and accumulation of organic anions50. The organic anions and uric acid interfere with peritubular uptake of loop diuretic. The hypoalbuminemia in patients with cirrhosis increases volume of distribution of diuretic with impaired delivery to kidney. Therefore, in order to overcome diuretic resistance, a higher dose of diuretic is required in presence of CKD. The loop diuretics are the most common class of diuretic used in patient with CKD. In addition to fluid mobilization, loop diuretic also helps correct metabolic acidosis, hyperkalemia and hypertension if present45. It is important to understand the steep dose-response curves of loop diuretic for its optimal use. An escalating doses of loop diuretic should be used to identifythe dose-threshold and thereafter only minor increase in dose can be allowed, as greater increase in dose would fails to elicit more response because of ceiling effect50. A recent meta-analysis revealed that coadministration of albumin with furosemide has modest effect on overcoming diuretic resistance in hypoalbuminemic patients54. Some other steps to improve diuretic response would be correction of metabolic acidosis and hyperuricemia, appropriate restriction of fluid and salt intake and, avoidance of drugs that interfere with peritubular uptake of diuretic such as non-steroidal anti-inflammatory drug (NSAID), beta-lactam, sulfonamide, valproic acid and methotrexate etc50. Among loop diuretics, furosemide is predominantly eliminated by kidney while torsemide has predominant hepatic and some renal clearance55. Therefore, if kidney dysfunction is predominant problem, one can prefer to use torsemide over furosemide whereas furosemide should be preferred over torsemide in case of significant hepatic dysfunction. One must be aware that a high dose of loop diuretic in setting of renal dysfunction can cause deafness and tinnitus because of secretion of potassium-rich endolymph56. Combining spironolactone to a loop diuretic is often desirable in cirrhotic patients with ascites57. In patients with CKD, if spironolactone is considered, its dose should be adjusted with the goal to avoid hyperkalemia. The thiazides are deemed to be ineffective in patients with advanced CKD because of more proximal sodium reabsorption and less sodium being delivered to the distal tubule where thiazide diuretic acts58. The addition of clonidine to counter activation of the sympathetic nervous system has been found to enhance response of diuretic therapy; however, its effect in CKD patients is not known59.
Vaptans
Vaptans are vasopressin V2 receptor antagonist that inhibit renal reabsorption of free water. They may be considered in cirrhotic patients with CKD who have intolerance to or poor response to routine diuretics. Studies have found tolvaptan to be an effective and potentially safe drug for treatment of refractory ascites in patients with decompensated cirrhosis with efficacy rate of 89.7% overall and 77% in patients with co-existing type-2 HRS60. Tolvaptan significantly increases urine volume in CKD patients with liver cirrhosis without worsening of renal dysfunction61. However, its diuretic response gradually diminishes with deterioration of the CKD stage62. Tolvaptan can also be used for fluid management in patients with diabetic nephropathy63. Tolvaptan significantly increases the serum sodium levels in patients with hyponatremia. In 2013, the US FDA issued a warning for tolvaptan use because of the potential risks of hepatocellular injury observed during a clinical trial in patients with autosomal dominant polycystic kidney disease. However, this trial had used tolvaptan at much higher dose and for long period of time (120 mg/d for 3 years). No such adverse effect has been reported from trial in patients with cirrhosis. Tolvaptan appears to be safe when administered a lower dose (15-60 mg/d) over a short period (7-30 d).
Vasoconstrictors and Albumin
Treatment with vasoconstrictors (terlipressin or noradrenaline) together with IV albumin appear to have limited role in patients with CKD. Though, reversal of type-2 HRS (functional-CKD) occurs in many cases, recurrence after the withdrawal of therapy is very common. Furthermore, data about the impact of this treatment on patients’ outcomes are controversial. Few studies have evaluated the efficacy of terlipressin in small number of patients with type-2 HRS, and results have been equivocal64,65. Because of small number of type-2 HRS patients in published studies addressing the role of terlipressin, even meta-analysis could not give conclusive treatment recommendations66. In a recent study, 46% of treated patients showed reversal of type-2 HRS; however, relapse occurred in almost half of responders67. Also, reversal of type-2 HRS before LT does not appear to offer a signi?cant advantage over patients who are untreated or has a failed treatment before LT43. Relapse of HRS appears to be more common in type-2 HRS than in type-1 HRS.65,68. Therefore, most of the current guidelines do not recommend vasoconstrictor treatment in functional-CKD.
Midodrine hydrochloride
Midodrine is an orally available a1-agonist which increases effective circulating blood volume and renal perfusion by increasing systemic and splanchnic blood pressure in cirrhotic patients. Despite favorable effects on systemic and renal hemodynamics, studies on midodrine alone or in combination have shown conflicting results. In patients with type-2 HRS, midodrine has only mild beneficial effect on systemic hemodynamics, with no effect on renal hemodynamics69.
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
If drugs to control ascites are ineffective or contraindicated, patients require repeated large volume paracentesis (LVP). However, in presence of dysfunctional kidney, ascites often reaccumulate rapidly requiring frequent LVP. This, apart from adding inconvenience to patients, put them at risk various complications such as worsening circulatory dysfunction, infection and bleeding. TIPS is increasingly being used for treatment of patients with refractory ascites and functional renal failure. It lowers portal pressure, relieves ascites, and improves natriuresis and kidney function. There are currently limited data on the clinical outcomes and safety of TIPS in patients with advanced CKD. Lakhoo et al70 reported effect of TIPS in 17 cirrhotic patients with CKD where control of ascites was seen in 83% patients but at the cost of high (47%) incidence of new or worsening hepatic encephalopathy (HE). Michl et al71 reported improvement in renal function and reduction in frequency of paracentesis following TIPS in 10 cirrhotic patients with renal impairment. Three patients in this study had organic kidney disease. A recent systematic review and meta-analysis suggested a potential survival benefit of TIPS in patients with HRS but with a high incidence of HE (49%). The pooled short-term and 1-year survival rates after TIPS were 86% and 64% in type-2 HRS, respectively. In addition, there was improvement in renal function after TIPS in 83% of HRS patients72. To summarize, TIPS appear to be very effective in functional CKD, and limited available data also suggest its efficacy in organic-CKD. However, TIPS may increase incidence of HE, and it is contraindicated in patients with encephalopathy, cardiopulmonary disease, and liver failure.
Renal Replacement Therapy
The ideal time for commencing renal replacement therapy in cirrhotic patients with CKD is not known. Because of shared symptoms between two diseases and overestimation of GFR, even uremic symptoms are attributed to those of advanced cirrhosis73. The hemodynamic alterations of cirrhosis, in particular, reduced peripheral intravascular resistance, pose a challenge to maintaining hemodynamic stability during dialysis. During hemodialysis (HD), sudden decrease in in intravascular volume due to ultrafiltration often contribute to hypotension. In addition, thrombocytopenia and coagulopathy of cirrhosis increase risk of bleeding complications. Another problem of HD in cirrhotic patients is increased risk for developing encephalopathy due to sharp changes in blood osmolarity and electrolyte levels. Peritoneal dialysis (PD) may offer significant advantages in such patients by overcoming several problems associated with intermittent HD and also allowing periodic evacuation of ascitic fluid72,74. Few studies evaluating survival of the cirrhotic patient on PD have reported a modest survival rates of 8 months to 66 months75. Furthermore, the fear of increased risk of peritonitis with PD has not been clearly documented so far.
Concerns Related to Medications
As majority of drugs are metabolized and/or excreted by hepatorenal system, prescribing medicines in cirrhotic patients with CKD is a challenging task76. There are no evidence-based guidelines for the use of medication in such patients. Though most of the drugs can be used safely in patients with advanced cirrhosis, common drugs which need to be avoided or used with extra caution in such are: antituberculosis drug, azithromycin, trimethoprim-sulfomethoxazole, itraconazole, nitrofurantoin, NSAIDs, oral hypoglycemic agents, anticonvulsants, benzodiazepines and antidepressants. In presence of renal dysfunction, common drugs that require avoidance or extra-cautions are: aminoglycosides, NSAID, metformin, acyclovir, spironolactone, gadolinium contrast, lithium, cefepime, methotrexate, enoxaparin, etc. Furthermore, the dose of many antibiotics need adjustment in accordance with GFR. In patients with advanced cirrhosis, non-selective ß-blockers may increase mortality in patients with renal dysfunction and refractory ascites owing to their negative impact on the cardiac compensatory reserve77. Therefore, the use of ß-blockers should be avoided or used cautiously with close monitoring of blood pressure, serum sodium, and creatinine, in such patients. Also, treatment with nucleos(t)ide analogues for hepatitis B increases risk of lactic acidosis in presence of renal dysfunction.
Liver Versus SLK Transplantation
The functional-CKD is potentially reversible after LT. Small series have shown reversal of type-2 HRS in the majority of patients after LT alone42,43. However, a prolonged type-2 HRS may cause ischemia-induced irreversible tubular or glomerular damage which may not recover with LT. There remains a significant risk of CKD during the first year after transplantation even if HRS reverses. Because assessment of renal function may be difficult in patients with type-2 HRS, a renal biopsy should be considered, whenever possible for identifying parenchymal changes and to decide between LT and SLK transplantation. Role of GFR and evidence of parenchymal injury is important in making such decision. CKD patients with estimated GFR<40 ml/min using the MDRD-6 equation should be considered for SLK transplantation78. In patients with GFR around 40 ml/min, kidney biopsy should be considered to assess glomerular and interstitial changes, and if it shows >30% glomerulosclerosis and/or interstitial fibrosis, SLK transplantation should be considered78. Regarding outcomes, Singal et al reported outcome data of 2,606 patients who received SLK transplants during 2002 to 2011. The survival rates of liver graft, kidney graft, and patient at 5 years post SLK transplantation were 66%, 65%, and 69%, respectively for HCV, and for NAFLD patients, corresponding values were 72%, 72%, and 74%, respectively. However, the outcome data specific to CKD patients were not assessed in this study23. In conclusion, the incidence of CKD has considerably increased in cirrhotic patients over last decade due to increase in the risk factors as well as inclusion of functional-CKD in defining criteria. The available data on this condition is extremely limited. Future studies are needed to clarify many controversial issues on this subject. The important issues to be addressed would be refinement of diagnostic criteria in order to avoid overdiagnosis of CKD; identification of biomarkers of chronic kidney damage, development of better tool to estimate GFR and formulating management strategies based on phenotypic characteristics of CKD in cirrhosis.
References - Parke CY, Martin P, Bunnapradist S. Renal dysfunction in cirrhosis. Clin Liver Dis (Hoboken). 2015 Jun 24;5:150-153.
- Bucsics T, Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5:127–137.
- Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 2011; 60:702-709.
- Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015; 62:968–974.
- Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012; 82:516-24.
- Levey AS, de Jong PE, Coresh J, Coresh J, El Nahas M, Astor BC, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011; 80:17-28.
- Trawale JM, Paradis V, Rautou PE, Francoz C, Escolano S, Sallée M, et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int 2010; 30:725–732.
- Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, et al.Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014; 60:622–632
- Fagundes C, Pepin MN, Guevara M, Barreto R, Casals G, Solà E, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 2012; 57:267–273.
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016;11: e0158765.
- Wong F, Reddy KR, O’Leary JG, Tandon P, Biggins SW, Garcia-Tsao G, et al. Impact of Chronic Kidney Disease on Outcomes in Cirrhosis. Liver Transpl. 2019; 25:870-880
- Maiwall R, Rao Pasupuleti SS, Bihari C, Rastogi A, Singh PK, Naik V, et al. Incidence, Risk Factors, and Outcomes of Transition of Acute Kidney Injury to Chronic Kidney Disease in Cirrhosis: A Prospective Cohort Study. Hepatology. 2019 Jul 16. doi:10.1002/hep.30859.
- Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al, Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011; 140:488-496.e4.
- Salerno F, Cazzaniga M, Merli M, Spinzi G, Saibeni S, Salmi A, et al. Italian Association of the Hospital Gastroenterologists (AIGO) investigators. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011; 55:1241-8.
- Chen CY, Lin CJ, Lin CS, Sun FJ, Pan CF, Chen HH, et al. The prevalence and association of chronic kidney disease and diabetes in liver cirrhosis using different estimated glomerular filtration rate equation. Oncotarget. 2017; 9:2236–2248.
- De Souza V, Hadj-Aissa A, Dolomanova O, Rabilloud M, Rognant N, Lemoine S, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology. 2014; 59:1522-31.
- Choi YJ, Kim JH, Koo JK, Lee CI, Lee JY, Yang JH, et al. Prevalence of renal dysfunction in patients with cirrhosis according to ADQI-IAC working party proposal. Clin Mol Hepatol. 2014; 20:185–191.
- Péron JM, Bureau C, Gonzalez L, Garcia-Ricard F, de Soyres O, Dupuis E, et al Treatment of hepatorenal syndrome as defined by the international ascites club by albumin and furosemide infusion according to the central venous pressure: a prospective pilot study. Am J Gastroenterol. 2005; 100:2702-7.
- Kumar R. Hepatogenous diabetes: An underestimated problem of liver cirrhosis. Indian J EndocrMetab 2018; 22:552-9.
- Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J Hepatol. 2017 ;67:1274-1280.
- Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med.2014; 11:e1001680.
- Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis 2014; 64: 638-652
- Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014; 98:216–221.
- Kalaitzakis E, Rosengren A, Skommevik T, Björnsson E. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci. 2010; 55:467-75.
- De Broe ME, Gharbi MB, Zamd M, Elseviers M. Why overestimate or underestimate chronic kidney disease when correct estimation is possible? Nephrol Dial Transplant. 2017 ;32(suppl_2): ii136-ii141.
- Wetzels JF, Kiemeney LA, Swinkels DW Willems HL, den Heijer M. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007; 72: 632–637
- Hsu CY, Bansal N. Measured GFR as “gold standard”--all that glitters is not gold? Clin J Am Soc Nephrol.2011; 6:1813-4.
- Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016; 65:809-824.
- Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med. 1987; 82:945–952.
- Woitas RP, Stoffel-Wagner B, Flommersfeld S, Poege U, Schiedermaier P, Klehr HU, et al. Correlation of serum concentrations of cystatin C and creatinine to inulin clearance in liver cirrhosis. Clin Chem. 2000; 46:712–715.
- Skulzacek PA, Szewc RG, Nolan CR, Riley DJ, Lee S, Pergola PE: Prediciton of GFR in liver transplant candidates. Am J Kidney Dis. 2003, 42: 1169-1176.
- McGuire BM, Julian BA, Bynon JS Jr, Cook WJ, King SJ, Curtis JJ, et al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006; 144:735-41.
- Demirtas S, Bozbas A, Akbay A, Yavuz Y, Karaca L. Diagnostic value of serum cystatin C for evaluation of hepatorenal syndrome. Clin Chim Acta. 2001; 311:81–89.
- Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, et al. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002; 48:850–858.
- Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology (Baltimore, Md) 2014;59:1532–42.
- Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013; 38:345–54.
- Stevens LA, Schmid CH, Greene T. Li L, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009; 75:652–60.
- Krones E, Fickert P, Zitta S. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol 2015; 16:1–10.
- Niemann CU, Walia A, Waldman J, Davio M, Roberts JP, Hirose R, et al: Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl. 2009, 15: 1852-1860.
- Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, et al: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transpl. 2008, 23: 414-416.
- Xu TY, Zhang Y, Li Y, Zhu DL, Gao PJ. The association of serum inflammatory biomarkers with chronic kidney disease in hypertensive patients. Ren Fail 2014; 36:666–672.
- Rodriguez E, Henrique Pereira G, Sola E, Elia C, Barreto R, Pose E, et al. Treatment of type 2 hepatorenal syndrome in patients awaiting transplantation: Effects on kidney function and transplantation outcomes. Liver Transpl 2015; 21:1347–1354.
- Tan HK, Marquez M, Wong F, Renner EL. Pretransplant type 2 hepatorenal syndrome is associated with persistently impaired renal function after liver transplantation. Transplantation 2015; 99:1441–1446.
- Jouet P, Meyrier A, Mal F, Callard P, Guettier C, Stordeur D, et al. Transjugular renal biopsy in the treatment of patients with cirrhosis and renal abnormalities. Hepatology 1996; 24:1143–1147.
- Franz M, Hörl WH. The patient with end-stage renal failure and ascites. Nephrol Dial Transplant. 1997; 12:1070-8.
- Mannucci PM, Tripodi A. Hemostatic defects in liver and renal dysfunction. Hematology Am Soc Hematol Educ Program. 2012; 2012:168-73.
- Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008 ;3:1526-33.
- Gkamprela E, Deutsch M, Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Ann Gastroenterol. 2017;30:405–413.
- Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35:329–44
- Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol. 2002; 13:798-805.
- Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2017; 15:438-445.e5.
- Kim AJ, Lim HJ, Ro H, Jung JY, Lee HH, Chung W, et al. Liver cirrhosis leads to poorer survival in patients with end-stage renal disease. Korean J Intern Med.2016;31:730-8.
- Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003; 38:258–266.
- Kitsios GD, Mascari P, Ettunsi R, Gray AW. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: A meta-analysis. J Crit Care 29: 253–259, 2014
- Brater DC: Diuretic therapy. N Engl J Med 1998; 339: 387–395.
- Delpire E, Lu J, England R, Dull C, Thorne T: Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 1999; 22: 192–195
- Runyon BA; Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD): Management of adult patients with ascites due to cirrhosis. Hepatology 2004; 39:841–856.
- Loyd J, Wright P. Are thiazide diuretics an effective treatment for hypertension in patients with chronic kidney disease? J Okla State Med Assoc. 2008; 101:84-85.
- Lenaerts A, Codden T, Meunier JC, Henry JP, Ligny G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology. 2006; 44:844-9
- Zhang X, Wang SZ, Zheng JF, Zhao WM, Li P, Fan CL, et al. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20 :11400–11405.
- Tanaka A, Katsuno T, Ozaki T, Sakata F, Kato N, Suzuki Y, et al. The efficacy of tolvaptan as a diuretic for chronic kidney disease patients. Acta Cardiol. 2015; 70:217-23.
- Ikeda S, Ohshima K, Miyazaki S, Kadota H, Shimizu H, Ogimoto A, et al. Impact of chronic kidney disease on the diuretic response of tolvaptan in acute decompensated heart failure. ESC Heart Fail. 2017; 4:614-622.
- Sato E, Nakamura T, Amaha M, Nomura M, Matsumura D, Yamagishi H, et al. Effect of tolvaptan in patients with chronic kidney disease due to diabetic nephropathy with heart failure. Int Heart J. 2014; 55:533–538.
- Ghosh S, Choudhary NS, Sharma AK, Singh B, Kumar P, Agarwal R, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: A randomized pilot study. Liver Int 2013; 33: 1187–1193
- Alessandria C, Venon WD, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol2002; 14:1363-1368.
- Dobre M, Demirjian S, Sehgal AR, Navaneethan SD. Terlipressin in hepatorenal syndrome: A systematic review and meta-analysis. Int UrolNephrol 2011; 43: 175–184.
- Nguyen-Tat M, Jäger J, Rey JW, Nagel M. Terlipressin and albumin combination treatment in patients with hepatorenal syndrome type 2. United European Gastroenterol J. 2019; 7:529-537.
- Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol 2007; 47:499-505.
- Werling K, Chalasani N. What is the Role of Midodrine in Patients with Decompensated Cirrhosis?.Gastroenterol Hepatol (N Y). 2011; 7:134–136.
- Lakhoo J, Gunasekaran SS, Lokken RP, Lipnik AJ, Ray CE Jr, Bui JT, et al. Does advanced chronic kidney disease impact transjugular intrahepatic portosystemic shunt efficacy and safety? Acta Gastroenterol Belg.2017; 80:243-248.
- Michl P, Gülberg V, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Transjugular intrahepatic portosystemic shunt for cirrhosis and ascites: Effects in patients with organic or functional renal failure. Scand J Gastroenterol.2000 ;35:654-8.
- Song T, Rössle M, He F, Liu F, Guo X, Qi X. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis. 2018; 50:323-330.
- Howard CS,Teitelbaum I. Renal replacement therapy in patients with chronic liver disease. Semin Dial 2005; 18:212-6.
- Chaudhary K, Khanna R. Renal replacement therapy in end-stage renal disease patients with chronic liver disease and ascites: role of peritoneal dialysis. Perit Dial Int 2007; 28:113-7.
- Marcus RG, Messana J, Swartz R. Peritoneal dialysis in end-stage renal disease patients with preexisting chronic liver disease and ascites. Am J Med 1992; 93:35-40.
- Amarapurkar DN. Prescribing medications in patients with decompensated liver cirrhosis. Int J Hepatol. 2011; 2011:519526.
- Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010; 52:1017-22.
- Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant 2012; 12:2901–2908.
|