48uep6bbphidcol2|ID
48uep6bbphidvals|1899
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Biliary cystadenomas (BCA) are rare cystic neoplasms of the liver. These account for less than 5% of nonparasitic cysts of the liver1 and occur almost exclusively in middle-aged women. The size ranges from 1.5 to 35?cm.2,3 Other cystic lesions such as simple cysts, hydatid cysts, abscesses, hematomas, cystic metastases, and polycystic liver disease can often have a similar presentation that could probably explain a high likelihood of misdiagnosis.
Biliary cystadenomas were first described in 19434, and only a few hundred cases have been reported in the literature to date. These are usuallybenign lesions with the potential for malignant transformation.3,5,6 Developmentally, these may arise from either an aberrant bile duct or directly from a primitive hepatobiliary stem cell.1,7,8 The majority are intrahepatic (85%)6,7,9,10,11, few are extra hepatic5,7,12, and rarelythe origin may be from the gall bladder.9,10,12
The real challenge is to identify these lesions accurately in the preoperative setting as they closely mimic other cystic lesions of the liver, and the treatment varies in these various lesions. Classical features of biliary cystadenoma like enhancing septae or mural nodules are present only in a third of the patients. Moreover, preoperative imaging is inconclusive in differentiating between the benign form from the malignant form; hence these lesions should always be resected. The dilemma does not end here, as it is impossible to reliably distinguish a simple cyst or a hydatid cyst from a benign biliary cystadenoma intraoperatively. Such suspicious lesions are often subjected to deroofing, marsupialization, or partial resection that lead to a high rate of recurrence (>90%). A high index of suspicion is required in these situations. Preoperative imaging indicative of biliary cystadenoma, absence of daughter cysts, clear fluid within the cyst, and intraoperative frozen section from the wall of the cyst can help in deciding the extent of resection in such situations. Resection or enucleation of the cyst is the treatment of choice whenever biliary cystadenoma cannot be ruled out conclusively during intraoperative evaluation.
The present case seriesaims to emphasize the significance of radiological features in preoperative diagnosis of biliary cystadenomas, surgical management, and postoperative follow-up for recurrence.
Materials and Methods
After permission from the Institutional Ethical Committee, records of patients with biopsy-proven biliary cystadeno-mas treated between January 2010 and October 2017 at our institute were reviewed retrospectively. Patient records were reviewed for demographic characteristics,clinical presentation, radiological findings, past and present surgical details, and outcome of surgery in terms of morbidity and mortality. Preoperative diagnosis was based on the presence of one or more radiological findings using ultrasound or cross sectional imaging: internal septations, mural nodules, papillary projections, and cyst wall enhancement. The choice of surgicalprocedure was based on the site of the lesion and proximity to the hepatic hilum and major vessels. Liver resection was the preferred procedure, and enucleation was done when resection was not feasible due to proximity to hilum or major vessels. Postoperative complications and morbidity were recorded. Histopathological diagnosis of biliary cystadenoma was confirmed in all patients. Patients were screened forrecurrence by ultrasound abdomen. Quantitative data was reported as median with range, and binomial data as percentages.
Results
There were a total of 12 patients in the study, all of them being female. The median age of presentation was 38 years (Range: 20-55 years). The most common symptom was abdominal pain (100%), followed by abdominal fullness and abdominal mass (Table1). One patient had jaundice preoperatively due to tumor emboli in the left hepatic duct. The median duration of symptoms was 13 months (2-24 months).
Recurrent Cases and Interventions
Five patients had index surgery done elsewhere, in the form of deroofing of cyst for suspected hydatid cyst in four patients, and one patient had undergone cystojejunostomy for suspected simple cyst of the liver. All had a recurrence, which occurred in 3 patients within 6 months and in 2 patients after 6 months of the index surgery (median: 7 months (2-10 months)) Only one patient with recurrent giant cystadenoma had intervention in the form of percutaneous cyst fluid aspiration before the definitive surgery. Two patients in the recurrence group had cyst wall biopsy done during the initial surgery, which was reported as biliary cystadenoma. The interval between the diagnosis of recurrence to the definitive procedure varied from 1 to 3 months.
Routine blood investigations, including liver function tests and coagulation parameters, were within normal limits in all patients except onepatient who had obstructive jaundice due to tumor emboli in the left hepatic duct. Serum carbohydrate antigen 19-9(CA 19-9) was raised preoperatively in three patients (25%).Preoperative diagnosis was based on typical ultrasound and CECT findings that included one or more features like a multiloculated cyst with a well-defined capsule, internal septations, mural nodules, and solid papillary projections. (Table 1). Differential diagnosis of biliary cystadenoma was reported by the radiologists in 5 out of 7(71.4%) of new cases and 4 out of 5(80%) of recurrent cases.
Eight out of the 12 patients underwent liver resection: 3 central hepatectomies, 1 left hepatectomy, 4 non-anatomical resections of the involved segments, and 4 enucleations.One of the patients in the liver resection group had tumor emboli in the left hepatic duct, which was removed during the surgery, and hepaticojejunostomy was done. Enucleation was done when the lesion was close to the hilum and major vessels and at difficult locations (segment 8,4A,2). All 4 patients in the enucleation group had an intraoperative frozen section of the cyst wall to rule out malignancy. The diagnosis of intrahepatic biliary cystadenoma was confirmed by histopathology in all patients. All the specimens were subjected to histopathological. The cuboidal lining of the cyst with subjacent cellular compact ovarian-like mesenchymal stroma was demonstrated in all cases. The diagnosis was confirmed with immunohistochemistry (IHC), showing nuclear positivity for estrogen receptor (ER) and progesterone receptor (PR). There was no evidence of malignancy in any of these patients.
Median cyst size was 11 cm (range: 7-39 cms). The largest lesion measured was 39×28 cms, which is the largest biliary cystadenoma reported to date to the best of our knowledge. (Table 2)
Intraoperative blood loss ranged from 150-950 mL (median 350 mL). Immediate complications in the postoperative period occurred in 3 patients in the form of bile leak, which gradually subsided over a period of 7-14 days. None of these patients required endoscopic, radiological, or surgical intervention. One patient had postoperative cardiac arrest possibly due to air embolism and developed post cardiopulmonary resuscitationhypoxic-ischemic encephalopathy. None of the patients developed surgical site infections. In the long term complication, 1 patient developed adhesive subacute intestinal obstruction 2 years after central hepatectomy and underwent laparotomy and adhesiolysis for the same.
All the patients were followed up in OPD with ultrasonography (USG) of the abdomen to rule out recurrence. Initial ultrasound was done at three months after surgery and then every 6 months. The longest follow up was for seven years post-surgery, and the shortest was three months. None of the patients had a recurrence of cyst during a median follow-up of 24 months.
Discussion
Biliary cystadenomas constitute less than 5% of cystic space-occupying lesions of the liver. It occurs typically in middle-aged women presenting with abdominal pain, discomfort, fullness, and sometimes a palpable mass. Asymptomatic cases have been reported when the size is small.1,6,7,14 Rare presentations include jaundice,vomiting, anorexia, and weight loss.14,15 It can present acutely with pain abdomen due to intracystichemorrhage or rupture of the cyst and also fever due to secondary infection of the cyst.16 The near-exclusive occurrence in females and the associated increase in size during pregnancy and following oral contraceptives suggest hormonal dependence.1,2,13
In the present case series, abdominal pain was the most common symptom followed by abdominal fullness and mass abdomen. One patient had obstructive jaundice. Biliary cystadenomas are usually multiloculated, septatedcystic tumors, and comprise of two types: with and without ovarian-like stroma.1,5,8 Edmondson et al. initially defined BCAs in 1958 as multilocular lesions with ovarian-like stroma, but subsequently, BCAs without ovarian stroma were reported. The ovarian-like stroma consists of compact spindle-shapedcells and supports the epithelium and is often seen exclusively in women.1,7,16 Microscopically they have loculi, which are limited by a single layer of cuboidal or column are pithelium resting on a basement membrane with multiple polypoidal or papillary projections. Cystadenomas with ovarian stroma are consider edpremalignant with a good prognosis, while those without ovarian stroma have a higher risk of transformation to malignancy and have a poorer prognosis.6,8,9 The lesions with ovarian stroma closely resemble mucinous cystadenomas of the pancreas; hence biliary cystadenomas were recently redefined as a mucinous cystic neoplasm of liver (MCN-L) in the 2010 World Health Organisation (WHO) classification.31
The majority of biliary cystadenomas do not communicate with the bile ducts, but rarely it may be present.2,17 None of the patients in our case series had cysto-biliary communication. Intracystic fluid may be clear or mucinous.5,7 The presence of blood-stained intracystic fluid should raise the suspicion of cystadenocarcinoma. Rarely the fluid may bebile stained, purulent, or gelatinous.9 Differential diagnosis of cystadenomas includes simple liver cysts, parasitic cysts (particularly hydatid cyst), liver abscess, polycystic liver disease, biliary cystadenocarcinoma, and cystic metastases.9,10,14 In our series, 4 patients were misdiagnosed as hydatid cyst and 1 as simple cyst of liver and operated elsewhere.
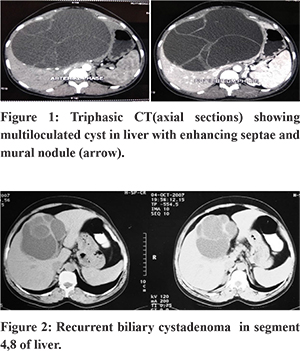
Preoperative diagnosis of biliary cystadenomas poses a challenge for both radiologists and surgeons as the symptoms are non-specific, and imaging findings may be similar to other cystic lesions of the liver. A high index of suspicion is required for accurate diagnosis in the preoperative setting. With considerable experience, it is often possible to make a diagnosis on imaging in the form of ultrasonography, CECT, and MRI abdomen (Figure 1,2). On all these imaging modalities, the presence of a multiloculated cyst, septations, and internal echoes, with papillary projections is typical.9,15 CECT also delineates the anatomic relation to surrounding structures, particularly major vessels.14,15,18,19 MRI can characterize the nature of fluid within the cyst9,18, that is blood or mucin. In spite of the advanced imaging modalities and aforementioned radiological features, the preoperative radiological diagnosticaccuracy may be as low as 30% leading to misdiagnosis20. Therefore a high index of suspicion is required in cases with a multiloculated, septate liver cyst, central location, recurrent cyst after previous partial resection. Although cross-sectional imaging can reliably differentiate cystadenomas from other cystic lesions of the liver, it may not be able to differentiate it from cystadenocarcinoma. An irregular thickness of the cyst wall, presence of mural nodules, or papillary projections are indicative of a malignancy.9,10,18,21 Hypervascularity of mural nodules on CT also suggests malignancy.22 Hence, the confirmation of the diagnosis is always by histopathological examination of the resected specimen. In our series, CT had shown multiple loculi and septations in all patients. Enhancing septae and mural nodules were seen in 6(50%) and 3(25%) patients, respectively. A preoperative core needle biopsy or FNAC to detect malignancy isnot recommended, especially in operable lesions due to low accuracy and the riskof needle seeding and dissemination.16,23,24 Elevated CEA and CA 19-9 in the serum or cystic fluid may aid inthe diagnosis and follow-up of patients.16,25 Pinto and Kaye, in their study, utilized intracystic CEA levels to differentiate BCA from benign cysts with 100% sensitivity and 94% specificity.26 A normal level, however, does not exclude a biliary cystadenoma; hence these procedures are not commonly done. In our series, only 3(25%) out of 12 patients had a raised preoperative CA 19-9 levels. The role of cyst fluid CEA, CA19-9, and serum tumor markers remains controversial as the sensitivity and specificity are not high enough to differentiate a biliary cystadenoma from cystadenocarcinoma.
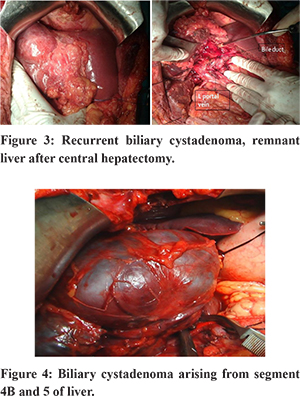
The important issues while diagnosing and treating a case of BCA are the incomplete excision of thecyst, misdiagnosis as a simple or a hydatid cyst, and the difficulty in differentiating cystadenocarcinomas, either pre or intraoperatively.1,5,9,12 Intraoperative frozen section of the cyst wall is a useful aid in such situations as it can differentiate a biliary cystadenoma from other cystic lesions of liver but not biliary cystadenocarcinoma. Historically, BCA’s have been treated with procedures like deroofing, Roux-en-Y cystoenterostomies, aspiration, or partial resection. However, these procedures have been associated with a high risk of recurrence7,9,14,27,28. Hence, complete resectionis the recommended treatment with negligible recurrence1,5,8,12 (Figure 3). Pinson et al. have reported cyst enucleation without late recurrence and mortality.29 This procedure is a validalternative where resection is difficult or is likely to be associated with higher morbidity.12,16
Laparoscopic resections of biliary cystadenomas have been described with similar morbidity and recurrence rates as open resection, the largest series being reported by Koffron et al. [30], who reported only 1 recurrence out of 22 patients who underwent a laparoscopic procedure for biliary cystadenoma. Whether the approach is open or laparoscopic, the aim should be complete resection of the lesion.
In conclusion, the diagnosis of biliary cystadenoma should be considered in any multilocular cystic lesion ofthe liver, especially recurrent cysts, particularly in a middle-aged woman. It is most commonly misdiagnosed for a hydatid cyst, especially in endemic regions, as seen in our series. Hence it should form the part of differential diagnosis of multiloculated cystic lesions of the liver. Complete resection of the cyst isrecommended when in doubt. The recommended treatment of choice for any suspected BCA is resection, as it is challenging to differentiate it from cystadenocarcinoma preoperatively. Enucleation is another feasible option and is indicated where resection is difficult due to the location of the tumor or proximity to the hepatic hilum and major vessels.(Figure 5)
References
- Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer.1985;56:1434–1445.
- Poggio PD and Buonocore M. Cystic tumors of the liver: apractical approach. World Journal of Gastroenterology. 2008; 14:3616–3620.
- Tsiftsis D, Christodoulakis M, Bree E, and Sanidas E. Primary intrahepatic biliary cystadenomatous tumors. Journalof Surgical Oncology. 1997; 64:341–346.
- Short WF, Nedwich A, Levy HA, and Howard JM. Biliary cystadenoma. Report of a case and review of the literature. Archives of Surgery.1971;102: 78–80.
- Ishak KG, Willis GW, Cummins SD, Bullock AA. Biliary cystadenoma and cystadenocarcinoma: report of 14cases and review of the literature. Cancer. 1977; 39: 322–338.
- Devaney K, Goodman ZD, and Ishak KG. Hepatobiliarycystadenoma and cystadenocarcinoma: a light microscopicand immunohistochemical study of 70 patients. AmericanJournal of Surgical Pathology.1994;18 :1078–1091.
- Lewis WD, Jenkins RL, McDermott WV. Surgical treatment of biliary cystadenoma. A report of 15 cases. Archives of Surgery. 1988 ; 123:563–568.
- Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. Journal of the American College of Surgeons. 2005 ;200:727–733.
- Manouras A, Markogiannakis H, Lagoudianakis E, Katergiannakis V. Biliary cystadenoma with mesenchymalstroma: report of a case and review of the literature. WorldJournal of Gastroenterology. 2006; 12:6062–6069.
- Palacios E, Shannon M, Solomon C, Guzman M. Biliary cystadenoma: ultrasound, CT, and MRI. Gastrointestinal Radiology. 1990; 15:313–316.
- Marcial MA, Hauser SC, Cibas ES, Braver J. Intrahepatic biliary cystadenoma. Clinical, radiological, andpathological findings. Digestive Diseases and Sciences. 1986;31:884–888.
- Thomas KT, Welch D, Pinson W. Effective treatment of biliary cystadenoma. Annals of Surgery. 2005 ;241, 769–775.
- Emre A, Serin KR, Uven KG. Intrahepatic biliary cysticneoplasms: surgical results of 9 patients and literature review. World Journal of Gastroenterology.2011 ;17:361–365.
- Florman SS and Slakey DP. Giant biliary cystadenoma:case report and literature review. American Surgeon. 2001;67: 727–732.
- Forrest ME, Cho KJ, Shields JJ, Wicks JD, Silver TM,Mc-Cormick TL. Biliary cystadenomas: sonographic- angiographic- pathologic correlations. American Journal of Roentgenology. 1980 ;135: 723–727.
- Dixon E, Sutherland FR, Mitchell P, McKinnon G, Nayak V. Cystadenomas of the liver: a spectrum of disease. Canadian Journal of Surgery. 2001; 44: 371–376.
- Zen Y, Fujii T, Itatsu K. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillaryneoplasm of the bile duct. Modern Pathology. 2006 ;19 :1243–1254.
- Federle MP, Filly RA, Moss AA. Cystic hepaticneoplasms: complementary roles of CT and sonography. American Journal of Roentgenology. 1981;136: 345–348.
- Koroglu M, Akhan O, Akpinar E, Oto A, Gumus B. Biliary cystadenoma and cystadenocarcinoma: two rare cysticliver lesions. Journal Belge de Radiologie. 2006 ;89: 261–263.
- Choi BI, Lim JH, Han MCl. Biliary cystadenomaand cystadenocarcinoma: CT and sonographic findings. Radiology. 1989 ; 171: 57–61.
- Pojchamarnwiputh S, Chiangmai WN, Chotirosniramit A,Lertprasertsuke N. Computed tomography of biliary cystadenoma and biliary cystadenocarcinoma. SingaporeMedical Journal. 1989. 49, 392–396.
- Sato M, Watanabe Y, Tokui K . Hepatobiliary cystadenocarcinomaconnected to the hepatic duct: a case report andreview of the literature. Hepato-Gastroenterology. 2003; 50, 1621–1624.
- Hai S, Hirohashi K, Uenishi T. Surgical management of cystic hepatic neoplasms. Journal of Gastroenterology. 2003 ;38 :759–764.
- Iemoto Y, Kondo Y, Fukamachi S. Biliary cystadenocarcinoma with peritoneal carcinomatosis. Cancer. 1981 ;48 :1664–1667.
- Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepaticbiliary cystadenoma: role of cyst fluid analysis and surgicalmanagement in the laparoscopic era.Surgery. 2004 ; 136 : 926–936.
- Pinto MM, Kaye AD. Fine needle aspiration of cystic liver lesions. Cytologic examination and carcinoembryonic antigen assay of cyst contents. Acta Cytol. 1989; 33:852–856.
- Teoh AYB, Ng SSM, Lee KF, Lai PBS. Biliary cystadenoma and other complicated cystic lesions ofthe liver: diagnostic and therapeutic challenges. World Journalof Surgery. 2006 ;0 : vol. 30, 1560–1566.
- Fiamingo P, VerouxM, Cillo U, Basso S, Buffone A, D’Amico DF. Incidental cystadenoma after laparoscopic treatment of hepatic cysts: which strategy?Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2004 ;14:282–284.
- Pinson CW, Munson JL, Rossi RL, Braasch JW. Enucleation of intrahepatic biliary cystadenomas.SurgeryGynecology and Obstetrics. 1989; 168: 535–537.
- Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluidanalysis and surgical management in the laparoscopic era. Surgery. 2004; 136:926–936.
- Tsui WMS, Adsay NV, Crawford JM, Hruban R, Kloppel G, Wee A, editors. WHO classification of tumors of the digestive system. 4. Lyon: WHO; 2010. pp. 236–8.