48uep6bbphidcol2|ID
48uep6bbphidvals|1887
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Pancreaticoduodenectomy (PD) is the treatment of choice for benign and malignant tumours located in the periampullary region and head of pancreas. Due to complexity of the anastomosis especially to the pancreas, there is possibility of life threatening complications following the leak which increases the morbidity and mortality. Incidence of postoperative pancreatic fistula (POPF) varies widely in the literature from 2%-27% for PD, which is due to different methods used to define pancreatic fistula1,2. International Study Group for Pancreatic Fistula (ISGPF) was formed in 2004 to frame a uniform definition of POPF as drain output of any measurable volume of fluid on or after day 3 with amylase content greater than 3 times that of normal laboratory serum amylase value after pancreatic resections. Grading of fistula into A, B, C is made depending on the clinical parameters noted post operatively in a retrospective manner1,3. POPF increases delayed gastric emptying (DGE), wound infection, intra abdominal infection, increases length of hospital stay and cost4.
ISGPF score is calculated retrospectively. Recently many studies have been conducted to highlight the inherent deficiencies in grading severity5. Some surgeons have abandoned the use of drains where as other have begun to remove drains early in the post operative period based on drain volume and amylase content. However little is known about the postoperative dynamics of change in drain amylase, lipase content and amount of drain output towards prediction of post operative pancreatic fistula.
We retrospectively analyzed the factors which are associated with POPF formation following PD. It was proposed in this study to estimate the incidence of POPF according to ISGPF criteria and to study the factors which are associated with formation of POPF.
Materials and Methods
Patient population
This is a retrospective analysis of prospective cohort of patients who have undergone PD for both benign and malingnant lesion of the periampullary and head of pancreas (HOP) from July 2013 to May 2014 in Apollo Hospital, Chennai, India. Study was conducted after approval by the Hospital Scientific and Ethical committee.
Perioperative parameters
Pre-operative hematological, biochemical tests and CECT abdomen were done. Endoscopic biopsy of lesion was done for ampullary and duodenal adenocarcinomas. Pre-operative biliary stenting was done based on the presence of cholangitis. Surgery was performed by 2 surgeons in the department of Surgical Gastroenterology in Apollo Hospital, Chennai, India. Type of anastomosis and technique were in accordance with surgeons’ preferences. Use of octreotide during the peri-operative and post-operative period and removal of drains in the post-operative period were also according to the discretion of the operating surgeon. Abdominal drains were placed in all cases on right flank. Blood loss was estimated by adding the suction output to the subtracted weight of dry from wet abdominal pad/gauze. Patients who underwent emergency PD and patients who had ongoing pancreatitis or cholangitis were excluded from the study.
Data collection
Data was collected prospectively in excel sheet format. Patient demographics included age, sex, diagnosis, pathology, pre-operative bilirubin levels, pre-operative stenting, CA 19-9 levels, co-morbidities, albumin levels, peri-operative administration of octreotide. Intra-operative variables like type of surgery either classical PD (CPD) or pylorus preserving PD (PPPD), intra operative octreotide, type of anastomosis either pancreaticogastrostomy (PG) or pancreaticojejunostomy (PJ), intra operative blood loss, bile culture, texture of pancreas were also recorded. Postoperative parameters included serum amylase and lipase, drain fluid amylase and lipase on postoperative day 1, 3, 5 and 7. Post-operative day 3 white blood cell (WBC) count and serum C reactive protein (CRP) levels were also recorded. Day of drain removal, imaging in post-operative period if done were recorded. Incidence of DGE, intra abdominal abscess, wound infection, sepsis, hemorrhage, need for reoperation, hospital stay and mortality were also noted.
Control group: Post pancreaticoduodenectomy patients whodeveloped no POPF or grade A POPF according to ISGPF criteria were considered ‘control’ group in this study.
Clinical group: Patients who developed grade B and grade C POPF according to ISGPF criteria comprised ‘clinical’ or clinically relevant POPF group.
Statistical methods
The data was entered into an MS-Excel spread sheet and statistical analysis carried out using SPSS version 11. All categorical variables were expressed as either percentage or proportion. All continuous variables were assessed for normality using Shapiro Wilks test and expressed as mean +/- standard deviation. Comparison of normally distributed continuous variables was done by independent‘t’ test or ANOVA based on number of groups. Comparison of non-normally distributed continuous variables was carried out by Mann Whitney U test or Kruskal wallis H test based on groups. All categorical comparison were performed by either Pearson chi square test or Fisher exact test based on number of observations. An area under the curve (AUC) greater than 0.8 was considered to be of high diagnostic accuracy. Receiver operating curve (ROC) was used to find out the best cut off value at an optimized accuracy with equal weight given to the errors of sensitivity and specificity for drain amylase. p value < 0.05 was considered as statistically significant.
Results
Patient demographics and preoperative parameters
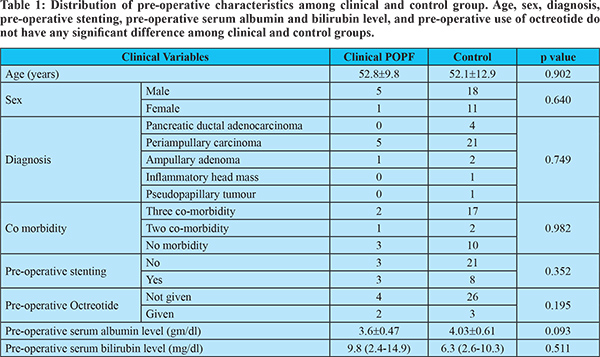
35 cases of PD were performed during the study period. Age of patients ranged from 28 years to 80 years with mean of 52 years, of which males formed 65.7% and females 34.3% in the group. Demographical parameters like age and sex, pathology, co-morbidity, preoperative albumin and bilirubin level and preoperative stenting incidence were not different in clinical POPF and control group (table 1). Periampullary tumours constituted 74.2% of cases which were malignant growths arising from distal bile duct, ampulla and duodenum. HOP adenocarcinoma constituted 11.4% of case, ampullary adenoma 3.6%, pseudopapillary tumour and inflammatory mass of HOP formed 2.9% each. Diabetes mellitus (DM), hypertension and coronary artery disease (CAD) or DM, hypertension and asthma were grouped into three comorbidity groups and they constituted 19 patients (54.3%). Patients who have two comorbidities like DM and hypertension or CAD and DM constituted 3 patients (8.6%) 13 patients (37.1%) had no co morbidity. No preoperative parameters were found to have impact on POPF.
Intraoperative parameters
Clinical POPF incidence was not different between CPD and PPPD but the type of pancreticoenteric anastomosis. PJ had lower incidence of clinical POPF than PG, though clinically not significant (p = 0.063). Clinical POPF did not vary significantly with pancreatic texture, use of peri-operative octreotide, positive bile culture and per-operative blood loss (table 1, 2).
Postoperative parameters
Pancreatic fistula according to ISGPF criteria was noted in 22 (62.8%) of patients in this series, but clinically relevant POPF i.e. ISGPF grade B (n = 2, 5.7%) and C (n = 4, 11.4%) occurred in 17.14% of patients. Morbidity and mortality rate in this series were 45.9% (n = 16) and 5.7% (n = 2) respectively. Day 1 drain fluid amylase (median: 485 U/L, range: 63-1200 U/L) varied significantly between clinical POPF and control groups (p = 0.013). Day 3 drain amylase level (median: 157.3 U/L, range 22-400 U/L, p = 0.022) revealed similar difference (table 3). Receiver operating curve (ROC) for day 1 drain amylase verified a cut off value of 968 U/L with 100% sensitivity and 79.3% specificity in predicting POPF (figure 1). At the same time day 3 drain amylase level >294 U/L had sensitivity of 83% and specificity of 75% in predicting POPF (figure 2). Drain fluid lipase and serum CRP levels failed to corelate with POPF (table 3). Peak raise in drain fluid amylase level was noted on the first day following surgery with maximum of 8180 U/L and a mean value of 1190 U/L. Maximum third day drain amylase level recorded was 7072 U/L and the mean value decreased to 591.4 U/L. Fifth day showed a significant decrease in the drain amylase level with maximum of 450 U/L and a mean value of 59.5 U/L (figure 3).
Clinical POPF was significantly associated with DGE, intra abdominal abscess, wound infection, sepsis, hemorrhage, need for reoperation, extended hospital stay and mortality (table 2, p = 0.0001). Two patients died and they all had clinical POPF.
Discussion
Risk factors for POPF are 1) patient-related risk factors like age, gender, jaundice, pre-operative biliary drainage and malnutrition; 2) disease-related risk factors like pancreatic pathology, pancreatic texture, pancreatic duct size, pancreatic juice output; 3) procedure related risk factors like operation time, resection type, anastomotic technique, intraoperative blood loss and other factors like surgeons’ experience, prophylactic use of octreotide and pre-operative stenting.
Patient-related risk factors
Patient characteristics are considered as predictive factors for POPF and include male sex, advanced age > 70 yrs, identifiable jaundice and low creatinine clearance (CCl)6. Age > 70 years had been found to have poor anastomotic healing and higher association of coronary artery and atherosclerotic diseases impairing visceral perfusion, ultimately leading to POPF6. Age and sex of the patients did not significantly affect the outcome in the present study (p = 0.563 and p = 0.64 respectively). Jaundice rather than CCl has been previously reported to be significant patient-related risk factor predisposing to POPF. Pre-operative serum bilirubin level had no significant impact on fistula development in present study similar to the observation by Yeh T.S et al who pointed duration of jaundice rather than the extent of jaundice to be of more importance7. Pre-operative biliary decompression as well had no impact on POPF (p = 0.352, table 1). Lower CCl is known to precipitate acute renal failure, intra-abdominal bleeding, sepsis and subsequent POPF particularly in those with obstructive jaundice. Fortunately no patient operated in this study had high preoperative creatinine value and was therefore investigated for CCl. Interestingly DM and neoadjuvant chemoradiotherapy have been shown to offer a protective benefit against pancreatic fistula, with the latter presumably causing a decrease in pancreatic exocrine secretion. Increase in number of co-morbidities has an additive effect in studies by Pratt et al8 but this study failed to show association with clinically relevant POPF (P=0.982). No patient received neoadjuvant treatment.
Pancreas and disease-related factors
The most widely recognized risk factors for pancreatic fistula are directly linked to state and disease of the pancreas and periampullary region. Principal among them is a soft pancreatic parenchyma. In a series of nearly 2000 PD, it is noted that a soft pancreas is associated with 22.6% fistula rate and lead to a 10-fold increased risk of POPF versus an intermediate or hard gland6,9. That a fibrotic pancreatic remnant facilitates anastomotic integrity is also well accepted10. This study failed to show any impact of pancreatic texture on POPF development following PD. Also it did not reveal any association of POPF with pancreatic disease contrary to the finding of its higher incidence in duodenal adenocarcinoma, ampullary lesions, cystic lesions of pancreas in some studies11.
Operative risk factors
In past two decades, various technical aspects have been scrutinized to identify operative factors associated with increased fistula rate. Various techniques for managing the pancreatic remnant have been compared including PG vs. PJ, the duct to mucosa vs. invagination pancreaticojejunal anastomosis, stent vs. no stent across the pancreaticoenteric anastomosis, single vs. double Roux-en-Y loop reconstruction and the use of octreotide and fibrin sealants 10. Increased intra-operative blood loss is an important risk factor for developing POPF. Yeh et al group noticed in their study that POPF group significantly suffered greater blood loss, rather than their no fistula counter parts (1584±862 mL versus 794±387 ml, p = 0.0005)7. They proposed that blood loss = 1,500 ml to be at higher risk of fistula development. Increased blood loss is likely to be associated with other factors including more advanced stages of disease, i.e. portal or superior mesenteric vein invasion, obesity, jaundice-associated coagulopathy and concurrent pancreatitis. Lai et al. found intra-operative blood loss = 1000 ml be significantly associated with POPF12. Only 4 patients in present study had = 1000 ml blood loss during surgery and thus failed to appreciate any significance in developing POPF (p = 0.123).
Waugh and Clagett first performed PG in clinical practice in 194613. PG has recently gained favor as a possible mean of reducing the incidence of POPF by several factors like acidic inactivation of pancreatic juice, absence of enterokinase in stomach, which is required for conversion of trypsinogen to trypsin and subsequent activation of proteolytic enzymes. A lack of enzyme activation may help in preventing autodigestion of the anastomosis. Furthermore the proximity of the pancreas to the posterior gastric wall allows potentially less tension on the anastomosis. The excellent blood supply of stomach favors anastomotic healing and the thickness of the stomach holds the sutures well10.
On the other hand PJ has been the most commonly used method of pancreaticoenteric anastomosis after PD. The jejunum is a logical choice for a pancreaticoenteric anastomosis due to its generous blood supply and mobile mesentery. Yet during the past 30 years, this technique has consistently been reported to yield on an average a 10% fistula rate (range 2%-19%)10. Apart from the different positions of the jejunal loop (antecolic, retrocolic, or retromesenteric) and other variations, such as isolated Roux Loop PJ, the anastomosis can be performed as an end-to-end anastomosis with invagination of the pancreatic stump in the jejunum or as end to side anastomosis with or without duct to mucosa suturing. In addition to these, there are some more variations of these anastomotic techniques. Duct to mucosa pancreaticojejunal anastomosis is theoretically more rational technique to avoid POPF for its direct contact of the pancreatic duct with jejunal mucosa, preventing direct contact of the pancreatic juice with the cut end of the pancreas. Though it seems difficult in undilated ducts, in recent years this technique has been preferred regardless of the diameter of the pancreatic duct12. Suzuki et al. selected various PJ techniques according to the pancreatic texture and duct size and obtained an overall pancreatic leakage rate of 8%14.
Several meta-analyses comparing PG and PJ found less POPF with PG but similar overall complications including mortality in both groups15-18. Grendar J et al in a recent randomized controlled trial (RCT) found no superiority of PG over PJ19. In another RCT comparing PG with PJ, Yeo et al found similar fistula rates of 12% in both groups but more clinically relevant POPF in the PG group (4 out of 10, p = 0.043)20. In present study PG has been found not to be significantly associated with clinical POPF compared to PJ. Also type of surgery either CPD or PPPD did not have any significance in developing POPF (p = 0.546).
Clinical course
ISGPF criteria is useful for reporting and comparing the incidence and severity of POPF uniformly among different canters, but its retrospective character does not help predicting the clinical course and clinical decision making in managing the POPF21. In this study Grade A fistulas constituted 45.7% of patients. The critical question in managing these patients is to know, how to identify patients who may develop complications requiring interventions and who can be managed without complications. Very little data are available to predict factors that allow identification of patients, at the time of onset of fistula, as having “high risk” or “low risk” fistula. To identify factors which are associated with high risk fistula, variables studied, were day 1 drain fluid amylase, lipase; day 3 drain fluid amylase, lipase, CRP levels, WBC count; day 5 drain fluid amylase, lipase, CRP levels and day 7 drain fluid amylase and lipase.
Day 1 drain fluid amylase level was found to be significant in predicting high risk fistula in this study with a cut off value of 968 U/L as compared to higher cut off value of 5000 U/L in Molinari E et al series22. Similarly other studies by Sutcliffe et al showed cut off value of day 1 drain fluid amylase concentration 350 U/L and by Kawai et al and El Nakeeb et al, 4000 U/L each, predicting clinically significant POPF8,23-25. Whereas Moskovic et al found no usefulness of day 1 drain fluid amylase level in predicting clinical POPF26. Pratt et al observed latent development of POPF in 26.4% and clinically relevant POPF in 36% of patients, who had normal drain fluid amylase levels and this group of patients faced worst outcome27. Though the mean value of day 1 drain lipase levels was 2122.5 U/L which was comparatively higher than the mean value of day 1 amylase 1190 U/L, it was not associated with clinically significant POPF formation.
Day 3 drain amylase level was found to be significantly associated with predicting high risk POPF formation with cut off value of 294 U/L in our study. Day 3 CRP level was not associated with clinical POPF in this study contrary to Ansorge et al who found day 1 drain amylase, day 2 drain amylase and day 3 CRP levels to be significant for predicting clinically relevant POPF28. A study found increased CRP level in patients requiring reoperation and in patients with in hospital mortality5. Day 3 drain fluid lipase level (median: 174 U/L, range: 28-825 U/L, p = 0.2) was not found to be associated with clinically relevant POPF in this study as compared to another study which found lipase levels > 1000 U/L to be highly sensitive and specific for diagnosis of clinical relevant POPF22.
Pre-operative, intra operative or post-operative octreotide did not confer benefit for preventing POPF in our series (table 1,2).There are 11 RCTs involving 2023 patients in whom the somatostatin analogue was examined. Five RCTs from Europe and 1 RCT from Asia showed the benefit of perioperative use of somatostatin analogues to decrease the postoperative complication rate. On the other hand, 2 recent RCTs from Europe and 3 RCTs from USA failed to show benefit6,10. Connor et al. analyzed 10 studies and showed that somatostatin and its analogues reduced rate of biochemical fistula but not the incidence of anastomotic disruption29. Hence the prophylactic use of perioperative somatostatin and its analogues to prevent POPF after pancreatic surgery remains controversial. It does not result in reduction of mortality. However the efficacy of prophylactic octreotide is reported in selective administration in the setting of high risk glands, including patients with either soft glands or small pancreatic duct, in those harbouring ampullary, duodenal cystic or islet lesions, or in cases where intraoperative blood loss is excessive. Prophylactic octreotide did not influence clinically relevant fistula rates among low-risk glands.
Prevalence of PPH in this study was 14.2% which is higher compared to Yekebas et al (5.4%)30. Mortality due to PPH in this study is 2% as compared to higher mortality of 16% in Yekebas. et al series. PPH and other complications, particularly DGE (22.9%), ileus, wound infection, intra-abdominal abscess, pancreatitis, haemorrhage, and sepsis were significantly associated with POPF in this study (p = 0.0001). Nearly all the patients who developed late PPH (3 of the 4 patients) had POPF. Hospital costs, rate of reoperation, hospital stay and readmission were significantly increased.
Conclusion
Post-operative day 1 and day 3 drain fluid amylase >968 U/L and >294 U/L respectively were associated with clinically relevant POPF. This study was limited by small sample size; nevertheless it established the possibility of predicting clinically significant POPF on first postoperative day from simple drain fluid amylase estimation.
References
- Neoptolemos JP, Russell RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997; /84:1370-6.
- Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R,Falconi M, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004; /21:/54-9.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al. Post operative pancreatic Fistula: an International study group definition. Surgery. 2005;138(1):8-13.
- Callery M.P, Pratt W.B, Vollmer C.M. “Prevention and management of pancreatic fistula,” J GastrointestSurg. 2009;13( 1) 163–173.
- Gebauer F, Kloth K, Tachezy M, Vashist YK, Cataldegirmen G, Izbicki JR et al. Options and limitations in applying the fistula classification by the international study group for pancreatic fistula. Ann Surg. 2012 Jul; 256(1):130-8.
- Lin.J.W, Cameron.L, Yeo.C.J, Riall.T.S, Lillemoe.K. Risk factors and outcome in post pancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004; 8:951-989.
- Yeh.T.S, Jan.Y.Y, Jang.L.B et al. Pancreaticojejunalanastamotic leak after pancreaticoduodenectomy-multivariate analysis of perioperative risk factors. J Surg Res 1997; 67(2):119-125.
- Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32(3): 419-28.
- Van Berge Henegouwen MI, De Wit L.T, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors and treatment of pancreatic leakage after pancreaticoduodenectomy, Drainage versus resection of the pancreatic remnanat. J Am Coll Surg. 1997; 185(1):18-24.
- Norman Oneilmachado. Pancreatic fistula after pancreatectomy: Definitions, risk factors, preventive measures, and management-Review. Int J Surg Oncol. 2012; 1:1-10.
- Poon R.T.P, Lo S.K, Fang.D, Fan.S.T, Wong. J. Prevention of pancreatic anastamotic leakage after pancreaticoduodenectomy. Am J Surg. 2002; 183(1):42-52.
- Lai E.C.H, Lau S.H.Y, Lau W.Y. Measures to prevent pancreatic fistula after pancreaticoduodenectomy: a comprehensive review. Arch Surg. 2009;144(11):1074-1080.
- Waugh.JM and Clagett.OT. Resection of the duodenum and head of pancreas for carcinoma. An analysis of thirty cases. Surg. 1946; 20(2):224-232.
- Suzuki.Y, Fujino.Y, Tanioka et al. Selection of pancreaticojejunostomy techniques according to pancreatic texture and duct size. Arch Surg. 2002;137(5):1044-1048.
- Que W, Fang H, Yan B, Li J, Guo W, Zhai W et al. Pancreaticogastrostomy vs. pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials.Am J Surg. 2015; 209(6):1074-82.
- Zhang X, Ma L, Gao X, Bao H, Liu P, Aziz A et al. Pancreaticogastrostomy versus pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials.Surg Today. 2015 ; 45(5):585-94.
- Chen Z, Song X, Yang D, Li Y, Xu K, He Y.Pancreaticogastrostomy vs. pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis of randomized control trials.Eur J Surg Oncol. 2014 ; 40(10):1177-85.
- Xiong JJ, Tan CL, Szatmary P, Huang W, Ke NW, Hu WM et al. Meta-analysis of pancreaticogastrostomy vs. pancreaticojejunostomy after pancreaticoduodenectomy.Br J Surg. 2014 ; 101(10):1196-208.
- Grendar J, Ouellet JF, Sutherland FR, Bathe OF, Ball CG, Dixon E. In search of the best reconstructive technique after pancreaticoduodenectomy:pancreaticojejunostomy vs. pancreaticogastrostomy.Can J Surg. 2015 ;58(3):154-9.
- Yeo CJ, Cameron JL, Maher MA et al. A prospective randomized trial of pancreaticogastrostomy vs.pancreaticojejunostomy following pancreaticoduodenectomy. Ann Surg. 1995; 222:580.
- Yang Y.M, Tian X.D, Zhuang W.M, Wang Y.L, Wan, Huang Y.T. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2009; 11(16):2456-2461.
- Facy O, Chalumeau C, Poussier M, Binquet C, Rat P, Ortega-Deballon P. Diagnosis of postoperative pancreatic fistula. Br J Surg. 2012; 99(8):1072-5.
- Pratt WB, Callery MP, Vollmer CM Jr. The latent presentation of pancreatic fistula. Br J Surg. 2009 ; 96(6): 641-9.
- Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamani G et al. Amylase value in drains after pancreatic resections as a predictive factor for post operative pancreatic fistula. Results of prospective study of 137 patients. Ann Surg. 2007; 246(7):281-287.
- El Nakeeb A, Salah T, Sultan A et al. Pancreatic anastamotic leakage after pancreaticoduodenectomy , risk factors clinical predictors and management (single center experience) World J Surg. 2013;37:1405-1418.
- Moskovic DJ, Hodges SE, Wu MF, Brunicardi C, Hilesenbeck SG, Fisher WE. Drain data to predict clinically relavant pancreatic fistula. HPB. 2010;12(7):472-481.
- Strasberg SM, Linehan DC, Clavien PA, Barkun JS. Proposal for definition and severity grading of pancreatic anastomosis failure and pancreatic occlusion failure, Surg. 2007; 141(4):420–42.
- Ansorge C, Nordin J.Z, Lundell L, Strommer L, Rangelova E, Blomber J et al. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. Br J Surg. 2014; 101:100-108.
- Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta-analysis of the value of somatostatin and its analogues in readucing complications associated with pancreatic surgery. Br J Surg. 2005; 92(9):1059-1067.
- Yekebas EF, Wolfram L, Cataldegirmen G et al. Post pancreatectomy hemorrhage: Diagnosis and treatment-an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007; 246(2):269-280.