48uep6bbphidcol2|ID
48uep6bbphidvals|1732
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Portal hypertension in children usually presents with recurrent life threatening upper gastrointestinal (GI) bleeding and hypersplenism causing significant mortality and morbidity affecting the quality of life. Endoscopic sclerotherapy with banding is now treatment of choice for control of the upper GI bleed
1-5 However, in some children with extrahepatic portal hypertension, despite successful endoscopic control of oesophageal varices, recurrent and persistent episodes of upper GI bleeding may occur due to ruptured gastric varices or portal hypertensive gastropathy.
4,5 In addition, even after ablation of the varices with endoscopic therapy, in some children massive splenomegaly and hypersplenism may persist to cause further problems.
4,5 In these subsets, splenectomy with splenorenal shunt can be performed. In cases of portal hypertension in children due to extrahepatic portal vein obstruction with failed sclerotherapy, mesocaval, splenorenal, or Rex shunt are the preferred shunts.
5,6 However, a splenorenal shunt requires a patent splenic vein of adequate calibre with normal renal vein. Similarly, the creation of a Rex Shunt requires patent mesenteric and left portal veins. However, in patients with portal hypertension due to extensive cavernomatous transformation of the portal vein extending up-to the splenic vein and the superior mesenteric veins, it becomes technically challenging to do a conventional splenorenal shunt or a Rex shunt.
For such cases, we report a modification of the proximal splenorenal shunt procedure. It can be performed even in the presence of a cavernomatous malformation affecting the splenic or superior mesenteric vein because the described technique obviates the need of tedious splenic vein-pancreatic disconnection.
Methods
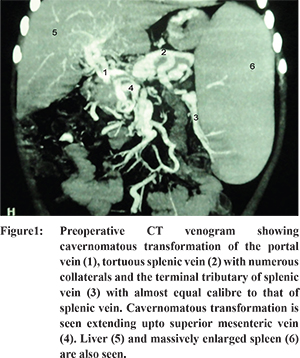
Ten boys and two girls with extrahepatic portal hypertension were included in the study from 2003-2010. Proper approval from the institutional review board (IRB) had been taken prior to the start of the study. Informed consent was taken from the parents of the children. Mean age of patient was 8.5 years (range 5-12 years). Indications of the procedure were: 1) Recurrent hematemesis referred after ablation of oesophageal varices with previous sclerotherapy and persistence of massive splenomegaly/hypersplenism reaching beyond umbilicus was found in eight patients of which three had concurrent ascites, 2) uncontrolled hematemesis during endoscopic sclerotherapy and massive splenomegaly in two patients, and 3) hematemesis with splenomegaly in two patients coming from remote areas who were not likely to return for follow up visits. Restoration of blood volume and correction of coagulopathy was performed for all patients. The children were then subjected to standard preoperative work-up revealing normal renal and liver function tests. Ultrasound abdomen with color doppler study and computerised tomography (CT) with angiography demonstrated morphology of extrahepatic portal vein system. All patients showed extensive cavernomatous transformation of portal vein, which extended up to the splenic and superior mesenteric veins in six patients (Figure 1). For these patients, we planned a modified shunt procedure that obviated the tedious splenic vein-pancreatic disconnection. The same procedure was done in the other six patients where the splenic vein was not distorted with a cavernoma and the size of splenic vein was almost equal to its widest terminal tributary. The calibre of the largest terminal tributary at the splenic hilum ranged from 6-7 mm in all patients. Splenectomy with careful harvesting of the terminal tributaries of splenic vein was planned, so that one of these could be anastomosed to either the left adrenal or the left renal vein with end-to-side anastomosis. All the shunts were performed by the same surgeon who had previous experience in performing conventional shunts.
Technique
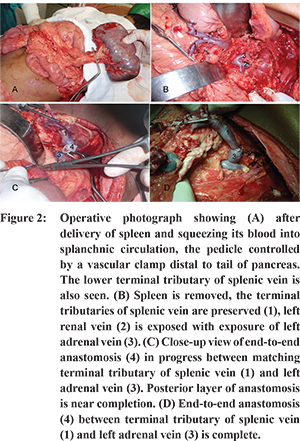
Wide exposure was obtained through a long transverse supra-umbilical incision corresponding to the level of the 12th rib. Without disturbing the massively enlarged spleen, a general survey of the abdominal cavity and viscera, including the liver was done, taking utmost care to avoid bleeding from the numerous tortuous and fragile collaterals. The lesser sac was entered after ligation and division of the gastrocolic omentum near the body of the stomach. Sessile short gastric vessels were ligated and divided. After ligation and division of lienocolic and lienophrenic ligaments and the accompanying venous collaterals, the massive spleen was isolated. It was handled with utmost care to avoid avulsion of the terminal branches of the splenic vein from the spleen. After mobilization of the spleen, the splenic artery was ligated and divided. The spleen was then gently and gradually squeezed to pump blood from its sinusoids into splanchnic circulation. The splenic pedicle was occluded with a vascular clamp just distal to the tail of the pancreas (Figure 2A). Distal to the clamp all three terminal tributaries of splenic vein were preserved up-to the subcapsular level and detached from the spleen one by one. The spleen was then removed leaving the long terminal tributaries of splenic vein intact. All the major tributaries were washed with heparinised saline and occluded individually with atraumatic bulldog vascular clamps. The clamp placed at the tail of the pancreas was released. The largest calibre tributary was selected for anastomosis. At subcapsular level, the venous wall was found to be very thin and delicate, and therefore, the terminal few millimetres of the selected tributary were discarded. Bleeds from minor tributaries were secured with ligation. The left renal vein along with left adrenal vein were exposed as described in literature (Figure 2B).7,8 If the calibre of left adrenal vein matched with the calibre of the selected tributary, as was the case in four patients, adequate length of the left adrenal vein was harvested for end-to-end splenoadrenal anastomosis using 6-0 prolene (Figure 2C & D). However, in the remaining eight patients with small sized left adrenal vein, left renal vein was prepared to perform an end-to-side splenorenal anastomosis. Through the other tributary of the splenic vein, a venous catheter was passed. It was connected to a manometer to measure the splenic vein pressure before the anastomosis was started and after the completion of anastomosis. Through the venous catheter placed in the other tributary of the splenic vein, the anastomosis was flushed with heparinised saline.
Out of four patients with end-to-end splenoadrenal shunt, 50% fall in splenic vein pressure was noted in three patients after completion of the splenoadrenal anastomosis. However, in one case, the end-to-end splenoadrenal shunt was inadequate for successful decompression of splenic vein pressure. The shunt was revised and converted into adequate calibre end-to-side splenorenal shunt between largest tributary of the splenic vein and the left renal vein.
The outcomes were assessed using following parameters; 1. intraoperative complications or difficulties, intraoperative shunt patency and fall in portal pressure, 2. postoperative complications in the form of recurrence of variceal bleeding, occurrence of ascites, postoperative encephalopathy, or occurrence of post splenectomy overwhelming sepsis, 3. late complications seen on follow up, and 4. shunt patency as seen on color doppler at 6-months interval after surgery and CT venogram on follow up at 1-year or later after surgery. All patients were given pneumococcal vaccine and long-term antibiotics as prophylaxis.
Results
Intraoperative complications
In one patient, a rent occurred in one of long terminal tributaries of splenic vein while dissecting so that minimal splenic vein-pancreatic disconnection had to be complemented to gain enough length for a tension-free anastomosis. In another patient, the end-to-end splenoadrenal shunt was found to be inadequate for successful decompression and had to be converted into end-to-side splenorenal shunt.
Intraoperative fall in portal pressure
Preoperative portal venous pressure ranged from 34-42 cm (mean=38.17 cm) of saline, measured via one of the tributaries of splenic vein. After completion of anastomosis, a 50% fall in pressure was noted in nine, while in the other three it was found to be only 20%. The anastomosis was patent in all.
Immediate postoperative complications
There was no shunt bleed. One patient developed pneumonitis, which was treated by intravenous antibiotics and chest physiotherapy. There was mild ascites in two, which resolved with conservative treatment.
Late complications
One patient had recurrent mild hematemesis three months after the surgery. On endoscopy, gastric ulcer was diagnosed. It was managed conservatively. Another patient presented at 1 year post-op with upper GI bleeding due to rupture of varices, as seen on endoscopy, and massive ascites. The ascites in this patient was relieved by conservative treatment and revision endoscopic sclerotherapy was done for variceal bleeding. Three years after the procedure, there occurred a recurrence of ascites in one of the three patients who had preoperative ascites, which was relieved on conservative treatment. No patient showed signs of post splenectomy sepsis. There was no occurrence of postoperative encephalopathy except in one case who presented with stunted growth and lack of concentration. This patient was previously treated for recurrent bleeding and ascites. Relief from hypersplenism was recorded in all patients presenting at follow-up. Out of two patients coming from remote areas, one patient had no symptoms after surgery on telephonic enquiry while the other patient was lost to follow-up.
Patency of shunt
Of the 12 operated patients, only ten presented for serial follow-up study with colour Doppler ultrasonography. At 6-months and 1-year, patent shunts were demonstrated in eight. CT venogram could be performed in eight patients (3 splenoadrenal, 5 splenorenal). All splenoadrenal shunts were found to be patent, while patency could be demonstrated only in four of the splenorenal shunts (Figure 3A & B).
Discussion
The ideal treatment for portal hypertension in children should be able to effectively decompress or eradicate bleeding gastroesophageal varices, resolve the problem of hypersplenism, ascites, and hepatopulmonary syndrome, yet be free from collateral risks or complications like portosystemic encephalopathy and growth retardation etc. Unfortunately, till date, no such treatment modality has been developed.
In children with portal hypertension due to extrahepatic portal vein obstruction with well-preserved liver function, endoscopic therapy in the form of sclerotherapy and banding has become mainstay of treatment for the control of variceal bleeding with excellent results reported by several centres.
1-4 However, despite successful endoscopic treatment and/or medication, few patients may still present with acute upper GI bleeding not amenable to control by either or even a combination of these. The causes of upper GI bleeding in this subset of patients may be manifold: Failure of endoscopic therapy, recurrent bleeding from gastric varices, inability of endoscopic therapy to control portal hypertensive gastropathy and portal hypertensive biliopathy.
4-6,9 Moreover, endoscopic banding and/or sclerotherapy is a long-term treatment requiring multiple sittings of endoscopy mandating multiple hospital visits. Endoscopy is also not capable of treating hypersplenism, growth retardation, portal hypertensive gastropathy, biliopathy, enteropathy and colopathy
4-6,9 and portopulmonary hypertension.
10,11 Therefore, in this group of patients, surgical intervention in the form of portosystemic shunt surgery as a one-time treatment becomes an important and effective option that can control bleeding from ectopic gastroesophageal varices, hypersplenism due to massive splenomegaly, portal hypertensive gastropathy, biliopathy, enteropathy and colopathy.
4-6,9 Improvement in growth parameters and quality of life has also been reported in children with portal hypertension after shunt surgery.
12,13 Among portosystemic shunt, selective mesocaval, splenorenal shunt or recently, a physiological, Rex shunt are mostly used shunt procedures for control of the bleeding gastroesophageal varices and hypersplenism.
5,6 However, in children with portal hypertension due to extensive cavernomatous malformation of portal vein extending up-to splenic and mesenteric veins, the creation of such a shunt may be technically challenging. Therefore, these options may not be preferred for effective decompression of gastroesophageal varices.
In our study group, the unique problem was extensive cavernomatous malformation of both portal and splenic veins along with significant hypersplenism and anemia. Therefore, we needed to create an effective shunt for decompression of bleeding gastroesophageal varices as well as to manage the severe hypersplenism.
The distribution of the terminal branches of both splenic artery and splenic vein have been described in literature.
14 The unique advantage with massive splenomegaly is that the terminal tributaries of splenic vein are stretched along with large spleen and thus length of terminal tributaries is considerably elongated with calibre almost equal to terminal splenic vein. Though, this cavernomatous malformation might extend to splenic vein and superior mesenteric vein, but terminal tributaries of splenic vein are free from disease and of adequate calibre, as demonstrated preoperatively on CT angiogram (
Figure 1). In literature, although splenoadrenal shunt as an alternative to splenorenal shunt has been described,
15-17 but, premeditated preservation of terminal tributaries of splenic vein and its use to make an anastomosis with either left adrenal vein or left renal vein has not been reported.
This technique has certain advantages: (1) There is no need to perform the delicate and difficult dissection of the splenic vein off the pancreas making this shunt a relatively easier procedure. (2) Because of the long semi-curved configuration of the upper or lower pole tributaries and upward and oblique course of left adrenal vein, the anastomosis shows gentle curvature without tension or kink of anastomosis reducing the risk of shunt block. (3) If after creation of splenoadrenal anastomosis, significant decompression of portal pressure is not achieved, then in the same operative field, this anastomosis can be converted into adequate sized end-to-side anastomosis between terminal tributary of splenic vein and left renal vein, as done in one case in the study group.
One major concern in the technique is the relatively small size of terminal tributaries of splenic vein theoretically raising the concern of increased incidence of shunt thrombosis and anastomotic stenosis. However, it has been established in literature that veins with diameter as small as 4-6 mm can effectively decompress bleeding gastroesophageal varices18. Further, the shunt was found to be patent without any anastomotic stenosis in 85% of patient (7 out of eight patient) in the study group on long-term follow-up with CT venogram. Furthermore, we hypothesise that a smaller of shunt may behave as a selective shunt similar to shunt described by Sarfeh,
18 but this could not be objectively determined. Another possible limitation is that the terminal tributary of splenic vein is thin walled and delicate, therefore anastomosis is little tedious and should be done by surgeon with sufficient experience in vascular anastomosis. Splenectomy in children leading to sepsis was a major concern, but it was duly mitigated by the relatively low incidence of postsplenectomy sepsis in our country as compared to western population.
9 One possible explanation given for this is that, in the developing countries, the children are exposed to bacterial infection from birth, which may, to some extent, provide protective immunity.
As most of the children with portal hypertension come with massive splenomegaly and symptomatic hypersplenism, therefore, removal of spleen is warranted rather than its preservation in these children. In the era of partial splenectomy, preservation of small segment of upper pole of spleen while discarding remaining part of spleen yet using the lower tributary for anastomosis has been reported in literature.
19
Conclusion
In children with extrahepatic portal hypertension, presenting with massive splenomegaly and upper GI bleeding, premeditated preservation of terminal tributaries of splenic vein during splenectomy can obviate or minimise the need of complex splenic vein dissection off the pancreatic bed and its anastomosis with left adrenal or left renal vein can decompress the portal hypertension.
References
- Gonclave ME, Cardoso SR, Maksoud JG. Prophylactic sclerotherapy in children with esophageal varices: long term results of a controlled prospective randomized trial. J Pediatr Surg. 2000;35:401-405.
- Krige JE, Bornman PC. Endoscopic treatment of oesophageal varices. S Afr J Surg. 2000; 38: 82-88.
- Celinska-Cedro D, Teisseyre M, Woynarowski M, Socha P, Socha J, Ryzko J. Endoscopic ligation of esophageal varices for prophylaxis of first bleeding in children and adolescents with portal hypertension: preliminary results of a prospective study. J Pediatr Surg. 2003; 38:1008-1011.
- Zargar SA, Yattoo GN, Javid G, Khan BA, Shah AH, Shah NA, et al. Fifteen-year follow up of endoscopic injection sclerotherapy in children with extrahepatic portal venous obstruction. J Gastroenterol Hepatol. 2004;19:139-145.
- Poddar U, Borkar V. Management of extra hepatic portal venous obstruction (EHPVO): current strategies. Trop Gastroenterol. 2011;32:94-102.
- Shepherd RW. Portal hypertension in children. In Blumgart LH, Belghiti J, Jarnagin WR, DeMatteo RP, Chapman WC, Büchler MW, et al (eds). Surgery of the Liver, Biliary Tract, and Pancreas. 4th Edn, Philadelphia: Saunders Elseviers. 2007,1594-1600.
- Henderson JM. Distal Splenorenal Shunt. In: Blumgart LH, Belghiti J, Jarnagin WR, DeMatteo RP, Chapman WC, Büchler MW, et al (eds). Surgery of the Liver, Biliary Tract, and Pancreas. 4th Edn, Philadelphia: Saunders Elseviers. 2007,1641-1646.
- Karrer FM. Management of portal hypertension. In: Spitz L, Coran AG (eds). Operative Pediatric Surgery, 6th Edn, Hodder Arnold. 2006, 645-654.
- Rao KL, Goyal A, Menon P, Thapa BR, Narasimhan KL, Chowdhary SK, et al. Extrahepatic portal hypertension in children: Observations on three surgical procedures. Pediatr Surg Int. 2004;20:679-684.
- Silver MM, Bohn D, Shawn DH, Shuckett B, Eich G, Rabinovitch M. Association of pulmonary hypertension with congenital portal hypertension in a child. J Pediatr. 1992;120: 321-329.
- Tokiwa K, Iwai N, Nakamura K, Shiraishi I, Hayashi S, Onouchi Z. Pulmonary hypertension as a fatal complication of extrahepatic portal hypertension. Eur J Pediatr Surg. 1993;3: 373-375.
- Kato T, Romero R, Koutouby R Mittal NK, Thompson JF, Schleien CL, et al. Portosystemic Shunting in children during the era of endoscopic therapy: improved postoperative growth parameters. J Pediatr Gastroenterol Nutr. 2000;30:419-425.
- Menon P, Rao KL, Bhattacharya A Thapa BR, Chowdhary SK, Mahajan JK, et al. Extrahepatic portal hypertension: Quality of life and somatic growth after surgery. Eur J Pediatr Surg. 2005;15:82-87.
- Last RJ, Sinnatamby CS. Last’s anatomy-Regional & Applied. 11th edn, Edinburgh: Churchill Livingstone Elsevier. 2006,281
- Pujahari AK: Lieno-adrenal shunt. Trop Gastroentrol. 2006;27(3):136-137.
- Jovine E, Cescon M, Ercolani G, Masetti M, Mazziotti A, Cavallari A. Splenoadrenal shunt. An original portosystemic decompressive technique. Hepatogastroenterology. 2001; 48:107-108.
- Kulkarni VM, Nagral SS, Mathur SK. Use of adrenal vein conduit for splenorenal shunts: a case report. Hepatogastroenterology. 1999;46:2033-2034.
- Sarfeh IJ, Rypins EB, Conroy RM, Mason GR. Portacaval H-graft: Relationship of shunt diameter, portal flow pattern and encephalopathy. Ann Surg. 1983;197:422-426.
- Petroianu A. Treatment of portal hypertension by subtotal splenectomy and central splenorenal shunt. Postgrad Med J. 1988;64: 38-41.