|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
GIST, imatinib, gastrointestinal stromal tumor |
|
|
|
Nida Iqbal1, Atul Sharma1, Nk Shukla2, BK Mohanti3, SVS Deo2, Peush Sahni4, Sujoy Pal4, Sushmita Pathy3, Vinod Raina5, Lalit Kumar1
Departments of Medical Oncology1,
Surgical Oncology2,
Radiation Oncology3,
Gastrointestinal Surgery4,
Anaesthesia5 , Dr. B.R.A. Institute
Rotary Cancer Hospital 1-3 ,
All India Institute of Medical
Sciences1-4, Fortis Memorial
Research Institute3,5
Indraprastha Apollo Hospital6 ,
New Delhi, India.
Corresponding Author:
Dr. Atul Sharma
Email: atul1@hotmail.com
DOI:
http://dx.doi.org/10.7869/tg.278
Abstract
Background: Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract. We aimed to study the pattern of presentation and treatment outcome of advanced GIST patients seen by us in a 10- year period.
Methods: Medical records of GIST patients seen between years 2002-2012 were retrieved from institute as well as database maintained by authors. Patient included in this analysis had metastatic disease and unresectable and/or residual disease after surgery.
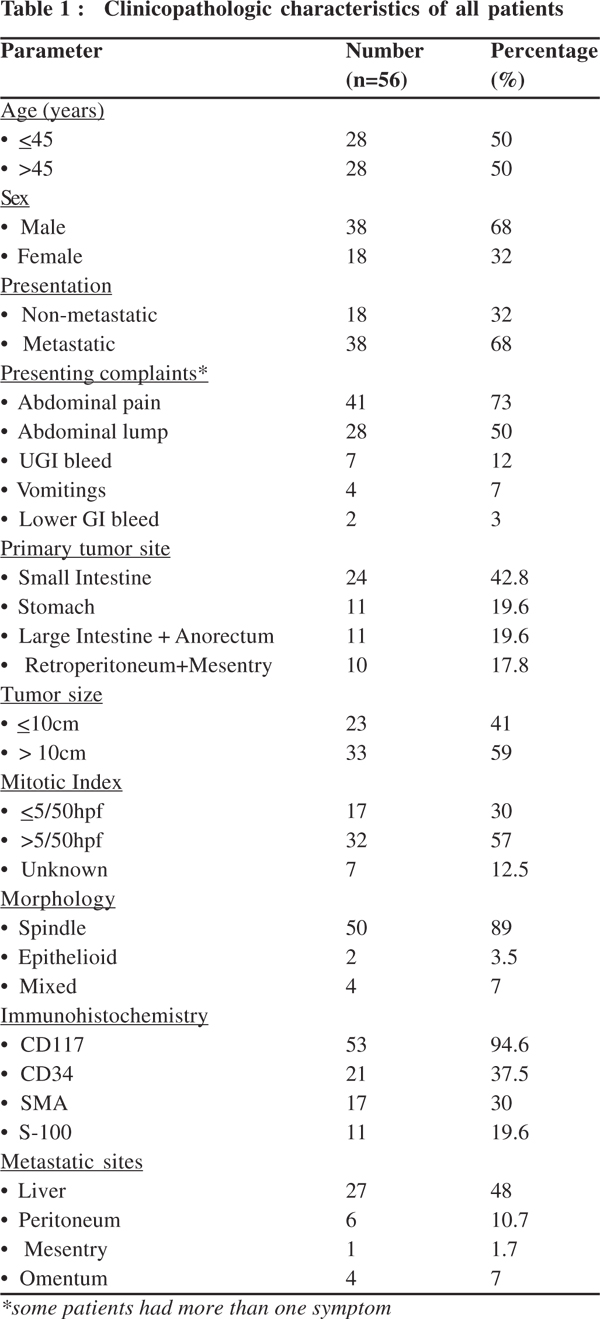
Results: During the study period 62 patients fulfilled the inclusion criteria but 6 were lost to follow up before treatment and hence 56 patients were analysed. Median age was 45.5 years (range 17-70 years) with a male female ratio of 2:1. Thirty eight (67%) patients had metastatic disease whereas 32% patients had unresectable or incompletely resected disease. The most common primary site was small intestine in 24 (42.8%) which was followed by stomach in 11 (19.6%) patients. The most common site of metastases was liver in 27 (48%) patients. Median tumor size was 12 cm (range 4-50 cm). Thirty two (57%) patients had mitotic counts of >5/50 HPF. All patients received imatinib. The most common response seen with imatinib was stable disease achieved in 29 (52%) patients. Imatinib was well tolerated by all patients without any drug discontinuation. The 5-year EFS and OS were 35% and 49%, respectively at a median follow up of 55 months. None of the patient or tumor factors were found to have prognostic significance in univariate survival analysis.
Conclusions: This is a single center experience of advanced GIST patients where small intestine was found to be the commonest disease site with imatinib producing disease stabilization in more than half of patients. Even though the survival was comparable to published reports, the major limitation was lack of mutation analysis.
|
48uep6bbphidvals|1342 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Gastrointestinal stromal tumors (GIST) are the most common mesenchymal neoplasms of the gastrointestinal tract accounting for <1% of all gastrointestinal tumors. GIST originate from interstitial cells of Cajal (ICC) or their stem celllike precursors which act as GI pacemaker cells.[1] The most commonly used marker for GIST is CD117 antigen which is an epitope of the KIT receptor tyrosine kinase and approximately 95% of GIST are positive for this antigen. The activating KIT mutations are seen in 85-95% of GIST. About 3-5% of ‘KIT - negative GIST’ contain PDGFR alpha mutations. Constitutive activation of either of these receptor tyrosine kinases plays a central role in the pathogenesis of GIST.[2,3] The high sensitivity and specificity of KIT is useful in differentiating GIST from other mesenchymal tumors of the gastrointestinal tract.[4] Surgery is the primary treatment of choice in localized or potentially resectable GIST.[5] Adjuvant imatinib is indicated in patients at high risk of recurrence.[6] In unresectable and metastatic disease, imatinib is the treatment of choice, continued indefinitely during disease progression.[7,8] For patients with progressive disease, imatinib dose escalation is considered in the absence of severe adverse drug reactions or switching to a second line tyrosine kinase inhibitor.[9] In this report, we aimed to review our experience with patients of advanced GIST and determine the clinic-pathologic factors, and treatment outcome with an emphasis on imatinib response and factors predictive of survival.
Materials and methods
This was a retrospective analytical study. Medical records of patients diagnosed with GIST and treated at our center were reviewed. Those who had metastatic disease and unresectable and/or residual disease after surgery (microscopic as well as macroscopic residual disease) were included in this study. Those with completely resected disease with negative microscopic margins were excluded from the analysis. All patients who presented with metastatic disease at our center were categorized under metastatic presentation, regardless of prior therapy. The primary tumor was evaluated by contrastenhanced computed tomography (CECT) and evaluation of metastatic disease by CECT chest, abdomen and pelvis. The diagnosis of GIST was established on the basis of histopathological examination and immunohistochemistry. The immunohistochemical profile was performed using a panel of CD117, CD34, vimentin, desmin, S-100, SMA and DOG-1. DOG- 1 was done only in a few patients due to recent incorporation in immunohistochemical profile of GIST. The histologic diagnosis of all tumors diagnosed outside our center was confirmed by members of the pathology department. The tumor characteristics included size, mitotic count and morphology. Mutation analysis for c-KIT and platelet-derived growth factor receptor-a (PDGFRA) was not performed since this was not available at our center.
Imatinib was given to all patients at a recommended dose of 400mg/day. Most of the patients received imatinib as part of GIPAP (Gleevec International Patients Assistance Program). Dose escalation of imatinib (600mg/day or 800mg/day) or second-line therapy with sunitinib was considered in patients who suffered disease progression on imatinib 400 mg. In patients who were intolerant to standard doses of imatinib, dose was reduced to 300 mg/day. Response to imatinib was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Complete Response (CR) was defined when there was disappearance of all target lesions. Partial Response (PR) was defined when there was at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum of longest diameter. Stable disease (SD) was defined when there was neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease and progressive disease (PD) was defined when there was at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions.
Survival analysis and statistics
Patients, tumor and treatment variables were analyzed for their relation to outcome. Any event and death from any cause were used as the end points in this study. Survival was estimated by the Kaplan-Meier method and compared using the log-rank test. Data was censored on 31 January 2014. Event-free survival (EFS) was calculated from the date of diagnosis to the date of disease relapse or progression or death from any cause. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause. The relationship between patient, tumor, and treatment characteristics to outcome were tested by univariate analysis using log-rank. P value of <.05 was considered statistically significant. Patients who were lost to follow-up were included in EFS and OS analyses, and outcome in these patients was confirmed by telephonic contact. STATA/ SE 11.0 (StataCorp LP, College Station, TX) was used for statistical analysis.
Results
Clinicopathologic characteristics
During the study period, 62 patients fulfilled the inclusion criteria but 6 were lost to follow up and data from 56 patients were analyzed. The clinicopathologic characteristics of all patients are summarized in Table 1. Median age was 45.5 (range 17–70) years with a male: female ratio of 2:1. Twenty-six patients had undergone surgery before coming to us and 7 had microscopic residual (R1) and 19 had gross residual disease (R2). Median size of the tumor was 12 cm (range 4-50 cm). Thirty-three (59%) patients had tumor size over 10 cm.
Treatment and response
All patients were initiated on 400 mg of imatinib daily. Nine (16%) patients achieved CR (2 with imatinib alone and 7 patients who had R1 resection). Nine (16%) patients had partial response. Disease stabilization was seen in 29 (52%) patients and 6 (11%) patients progressed on therapy. Thus, overall 47(84%) patients had either some response or disease stabilization.
Toxicity
Imatinib was well tolerated by all patients. Most common adverse effects were nausea, fatigue and facial puffiness. Four patients required dose reduction mainly because of dyspeptic symptoms. None of the patients required discontinuation of therapy.
Progression of disease
The disease progressed/recurred either upfront or following partial response to imatinib 400mg in twenty-six (46%) patients. Imatinib 600 mg was given to 24 patients and sunitinib to 2 patients. Partial response was seen in 3 patients and 8 patients had stabilization of disease.
Survival
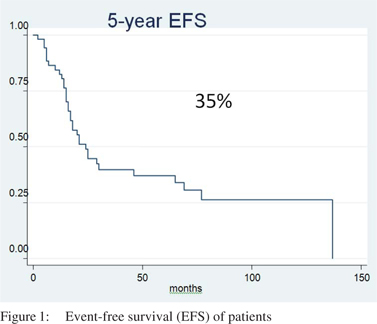
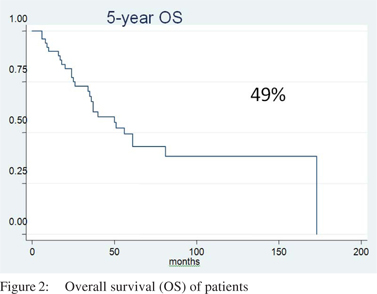
Median follow up was 55 months. The 5-year EFS and OS was 35% and 49%, respectively with a median EFS and OS of 22 months and 55 months, respectively (Figures 1 & 2).
Analysis of prognostic factors
The prognostic impact of baseline characteristics on survival was not identified in univariate analysis (Table 2).
Discussion
Due to rarity of GIST, there is limited published literature on clinical profile and treatment outcome in Indian population. Median age in our cohort was 45.5 years, which is consistent with published literature,[10-12] however, we observed small intestine as the commonest site of disease. This site distribution differs from what has been reported in Western literature,[13-16] but is consistent with some studies reported from India.[17,18] Our center being a tertiary care center, this indiscrepancy in site might be explained by referral bias. GIST arising from mesentry and retroperitoneum generally referred to as “extra-gastrointestinal stromal tumors (EGIST)” constituted 17.8% of patients in our cohort. This frequency is higher than the< 10% as is generally known for EGIST.[19] The presentation of GIST with abdominal pain, abdominal lump and upper gastrointestinal hemorrhage in our study is consistent with literature.[20,21] DOG1 (Discovered On GIST 1) antigen has recently been incorporated in the immunohistochemical panel, which is considered to have a higher specificity and sensitivity.[23]This was also reflected in our study as 100% of 18 patients, in whom DOG1 was done, showed positivity for this antigen.
Imatinib has revolutionized the treatment of GIST and is considered a frontline drug for patients with unresectable and metastatic disease. With imatinib treatment, 83%–89% of patients either respond or achieve stable disease whereas only 11%–17% progress. The 2-year survival of patients with advanced disease has risen to 75%–80% following imatinib treatment.[7,24,25] Even after imatinib dose escalation in patients with progressive disease, about a third of patients achieve stable disease and 2% respond.[26] Sunitinib is considered in patients who are imatinib resistant or develop intolerance to imatinib.[9] Surgery may be added to medical therapy for selected patients with GIST however its benefit in metastatic disease has not been shown.[27] Imatinib helped disease stabilization in more than half the patients with advanced GIST in our study with 2-year survival approaching 77% which is similar to that reported,[7,24] however the major limitation in our study was the lack of mutation testing for KIT and PDGFRA and access to sunitinib due to limited resources in developing countries.
Conclusion
Data of advanced GIST were collected from a tertiary referral hospital in North India where patients are referred from mainly Northern and Eastern parts of country. In this report GIST was found to be more prevalent in the small intestine. Imatinib therapy was associated with better disease control with almost half the patients achieving stable disease. Imatinib was well tolerated by all patients with only a few requiring dose reductions.
References
- Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–69.
- Kang HJ, Koh KH, Yang E, You KT, Kim HJ, Paik YK. Differentially expressed proteins in gastrointestinal stromal tumors with KIT and PDGFRA mutations. Proteomics. 2006;6:1151–7.
- Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC. KIT-negative gastrointestinal. stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–94
- Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–34.
- Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol.2005;16:566–78.
- Joensuu H. Adjuvant therapy for high-risk gastrointestinal stromal tumour: considerations for optimal management. Drugs. 2012;72:1953–63.
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34.
- Blay JY, Le CA, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–13.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
- Miettinen M, Hala M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: A clinicopathological, immunohistochemical and molecular genetics study of 906 cases before imatinib with long term follow up. Am J Surg Pathol. 2006;30:477–89.
- Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and growing adults. A clinicopathological, immunohistochemical and molecular genetics study of 44 cases with long term follow up and review of literature. Am J Surg Pathol. 2005;29:1373–81.
- Vasantha A. Lakshmi, Raju T. Chacko, Susy Kurian. Gastrointestinal stromal tumors: A 7-year experience from a tertiary care hospital. Ind J Pathol Microbiol. 2010;58:628–33.
- Trupiano JK, Stewart R, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors, a clinicopathological study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviour. Am J Surg Pathol. 2002;26:705–14.
- Rajappa S, Muppavarapu KM, Uppin S, Digumarti R. Gastrointestinal stromal tumors: a single institution experience of 50 cases. Indian J Gastroenterol.2007;26:225–9.
- Trent JC, Patel SR. Clinical updates on adult and pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23:xiii–xiv
- Lakshmaiah KC, Suresh TM, Babu G, Babu S, Purohit S, Guruprasad B et al.Gastrointestinal stromal tumors: A single institute experience from South India. Clinical Cancer Investigation Journal. 2014;3:62–5.
- Lamba M, Mukherjee G, Saini KS, et al: Clinico- pathologic pattern of gastrointestinal stromal tumors (GIST) in southern India: a single-institution experience. J Clin Oncol. 2007;25:1–20531.
- Attili SV, Ananda B, Mandapal T, Anjaneyulu V, Sinha S, Reddy OC. Factors Influencing Progression-Free Survival in Gastrointestinal Stromal Tumors With Special Reference to Pathologic Features, Cytogenetics, and Radiologic Response. Gastrointest Cancer Res. 2011;4:173–7.
- Dubey, Usha, Das, Rumpa, Agrawal, Asha, et al. Malignant extra gastrointestinal stromal tumours: what are the prognostic features to depend upon? Journal of Clinical and Diagnostic Research. 2011;52:369–71.El-Zohairy M, Khalil el-SA, Fakhr I, El-Shahawy M, Gouda I. Gastrointestinal stromal tumor (GIST)’s surgical treatment, NCI experience. J Egypt Natl Canc Inst. 2005;17:56–66.
- Miller TA. Leimyosarcoma. Not all gastric malignancies have a dismal prognosis. Gastroenterology. 1993;104:940–1.
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10.
- Lee C, Liang CW, Espinosa I. The utility of discovered on gastrointestinal tumor 1 (DOG1) antibody in surgical pathologythe GIST of it. Adv Anat Pathol. 2010;17:222–32.
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–5.
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren A, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–32.
- Hislop J, Mowatt G, Sharma P, Fraser C, Elders A, Jenkinson D, et al. Systematic review of escalated imatinib doses compared with sunitinib or best supportive care, for the treatment of people with unresectable/metastatic gastrointestinal stromal tumours whose disease has progressed on the standard imatinib dose. J Gastrointest Cancer. 2012;43:168–76.
- Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325–31.
|
|
|
 |
|
|