|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
Immunoglobulin G4, chronic liver disease, plasma cells, immunohistochemistry, autoimmune pancreatitis. |
|
|
Amarapurkar Anjali D1, Amarapurkar Deepak N2
Department of pathology1
TN Medical college,
BYL Nair ch Hospital & SRL Dr Avinash Phadke laboratory,
Department of Gastroenterology & Hepatology2,
Bombay Hospital & Medical
Research Centre, India
Corresponding Author:
Dr. Anjali D Amarapurkar
Email: anjali1963@gmail.com
DOI:
http://dx.doi.org/10.7869/tg.263
Abstract
Introduction: IgG4 disease has been characterised by lymphoplasmacytic inflammation, rich in IgG4 plasma cells, elevated serum IgG4 and clinical improvement with steroid therapy. There is limited information about IgG4 plasma cells in autoimmune hepatitis (AIH). Aim of this study was to determine IgG4 plasma cells in autoimmune hepatitis and its impact on clinical course and treatment outcome.
Material Methods: Liver biopsies from 40 patients with AIH before therapy were subjected to IgG4 immunostaining. Clinical history, liver function tests and response to immunosuppressive therapy were recorded. Patients were monitored for 4 weeks. Liver biopsy from 23 non AIH patients served as control. Depending on the presence of IgG4 plasma cells on immunohistochemistry, patients of autoimmune hepatitis were grouped into IgG4 positive (group A) and IgG4 negative (group B). Both groups were compared before and after immunosuppressive therapy for clinicopathological features.
Results: Tissue IgG4 plasma cells >5 per high power field (hpf) were seen in 10/40 (25%) and >10 per hpf in 4/40 (10%) cases of AIH. None of the cases from control group (non AIH) were positive for IgG4 plasma cells. Group A patients were significantly younger than group B. (p<0.05). There were no differences in histological severity but liver enzymes, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were significantly higher in group A than group B. Post treatment biochemical improvement was similar in both groups.
Conclusion: IgG4 positive AIH patients were younger with more abnormal liver enzymes. There was no difference in histology and response to treatment in both groups.
|
48uep6bbphidvals|722 48uep6bbph|2000F98CTab_Articles|Fulltext Autoimmune hepatitis is characterised by hypergammaglobulinemia, presence of liver related autoantibodies and liver biopsy showing plasma cells with interface activity.[1-8] Plasma cells are an important feature of autoimmune hepatitis. However their presence is not diagnostic of autoimmune hepatitis.[9] They can be seen in various liver diseases. The number of plasma cells in AIH is variable, ranging from mild to dense infiltration. They produce various subclasses of immunoglobulins such as IgG1, IgG2, IgG3 and IgG4, of which IgG4 is a unique antibody, is the least abundant IgG subclass, and accounts for less than 5% of the total IgG in healthy individuals.[10,11] Recently IgG4 disease was recognised as a fibro-inflammatory condition characterised by lymphoplasmacytic inflammation rich in IgG4 plasma cells, storiform fibrosis, elevated serum IgG4 concentration and clinical improvement with steroid therapy.[12-14] Tissue diagnosis of IgG4 plasma cells is considered the gold standard of this condition.[10-13] This disease was first recognised in relation to the pancreas and is termed as autoimmune pancreatitis. [15-17]
Many of these patients present with mass-like lesion in the pancreas, high level of serum IgG4 with inflammatory fibrosis around pancreatic duct rich in IgG4 plasma cells. It has been shown that there is remarkable response to steroids in these patients.[15-17] The commonest association of autoimmune pancreatitis is with IgG4 associated cholangitis or IgG4 related sclerosing cholangitis.[18-19] Autoimmune hepatitis which is the most common autoimmune liver disease shares some clinical and pathological features with autoimmune pancreatitis. There is limited information about the role of IgG4 plasma cells in autoimmune hepatitis. The majority of data related to IgG4 AIH is from Japan.[20-23] To our knowledge there are no reports of IgG4 AIH from India. Aim of this study was to determine IgG4 plasma cells in autoimmune hepatitis and its impact on clinical course and treatment outcome.
Material & methods
This was a prospective single center study carried out for the period of two years which was approved by the institutional ethic committee. Liver biopsies showing at least 10 portal areas from 40 patients with AIH before initiating therapy were included in this study. The diagnosis of AIH was done using simplified diagnostic criteria given by the International Autoimmune Hepatitis Group.[8] Liver biopsy specimens of 23 non-AIH patients consisting of 4 chronic HBV infection, 2 chronic HCV infection, 1 NASH and 16 normal liver histology cases of extrahepatic portal vein obstruction in which liver biopsy was normal served as control. HBV and HCV cases were selected in which liver biopsy showed at least focal plasma cells on haematoxylin and eosin stained sections. Clinical history, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and serum bilirubin levels were recorded in each patient. Serum immunoglobulin levels and liver related autoantibodies ANA, ASMA and LKM1 were done in all cases of AIH. The autoimmune hepatitis score was derived in each case. Score 6 was considered probable and 7 definite diagnosis of AIH.[8]
Serum IgG4 was assessed by standard technique (Nephelometry Siemens) with normal range between 0.03 to 2 g/L. Serum IgG4 > 2 g/L was considered abnormal. All 63 liver biopsies were processed routinely and stained with hematoxylin and eosin stain. They were analysed by an experienced hepatopathologist (first author). In each liver biopsy inflammation was graded semiqantitatively as mild, moderate and severe. Fibrosis staging was performed using Batts & Ludwig scoring system[24] as F0 (no fibrosis), F1 (increased portal fibrosis), F2 (periportal fibrosis), F3 (bridging fibrosis) and F4 (cirrhosis). In addition immunohistochemistry was performed using IgG4 antibody Zymed laboratories (10651USA) with dilution of 1:500 on all liver biopsies. Avidine biotin technique was used with diaminobenzidine tetrahydrochloride for visualization. This was followed by haematoxylin for nuclear counterstaining in all the cases. Appropriate positive and negative controls were used during immunohistochemical staining procedure. All portal areas were systematically scanned for presence of IgG4 plasma cells per 400X high power field (HPF). Portal areas with highest density of IgG4 plasma cells were selected. Average number of IgG4 plasma cells was counted in at least 5 portal areas. Biopsy was labelled as positive only when > 5 IgG4 positive plasma cells were identified per HPF as reported by Zhang et al.[25] IgG4 positivity in liver biopsy was considered a marker of IgG4 autoimmune hepatitis. Patients of autoimmune hepatitis were treated with prednisolone 30 mg/ day with tapering dose up to 10 mg/day and Azathioprine 50 mg/day for 2 years. All patients on treatment were subjected to liver function test at the end of 4 weeks and response to immunosuppressive therapy was recorded. Depending on presence of IgG4 plasma cells on immunohistochemistry, patients of autoimmune hepatitis were grouped into IgG4 positive (group A) and IgG4 negative (group B). Both these groups were compared before and after immunosuppressive therapy for clinicopathological features. Statistical analysis was carried out using SPSS, version 10.0(SPSS Inc, Chicago, IL, USA).
Results
Of the 63 cases, 40 were autoimmune hepatitis (30 definite and 10 probable diagnosis) and 23 were age and sex matched controls. All patients were between 19 and 70 (mean 47 ±7) years with male to female ratio of 1.1:1. Patients with autoimmune hepatitis were between 23 to 69 years (mean 48± 5 years) and male to female ratio was 1.2:1. ANA was positive (>1:80) in 17 (42.5 %) and ASMA in 5 (12.5%) cases. None of the cases were LKM1 positive. Serum immunoglobulin G > 1600 mg/dL was found in 35 (87.5%) cases. Autoimmune hepatitis score was 7 in 30 (75%) cases. Serum IgG4 was done in 20 of which raised levels were seen in 14 (70%) cases. On liver histology, moderate inflammation was present in 36 and sever in 4 cases of AIH. Inflammatory cells were mainly lymphocytes and plasma cells. Plasma cells were either equal or lesser than lymphocytes.
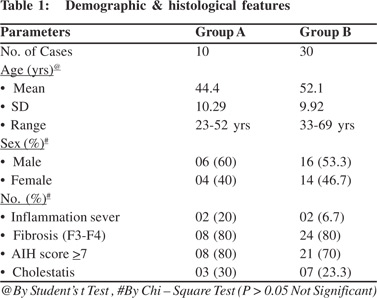
Significant fibrosis (F3, F4) was present in 18/40 (45%). In remaining 22, fibrosis was stage 1 to 2. Cholestasis was seen in 10/40 (25%) which was within cytoplasm of hepatocytes. It was mild in 7 and sever in 3 cases. There was no ductular cholestasis. Bile ductular proliferation was seen in 7/40 (17.5%) patients. On radiological investigations biliary tree was normal in all of them. Ultrasonography and CT scan or MRI did not show any pancreatic abnormality. None of these patients showed extrahepatic manifestations of AIH. Tissue IgG4 was positive (> 5 IgG4 plasma cells / hpf) in 10/40 (25%) while > 10 IgG4 plasma cells / hpf were seen in only 4 (10%) cases of autoimmune hepatitis (Figure 1). None of the cases from control group showed positivity for IgG4 positive plasma cells. Student’s t test and Chi square test was used to compare both groups. Mean age was 44.4±10.2 years in Group A, which was significantly less as compared to 52.1 years among Group B.
Male to female ratio was 1.5:1 in group A and 1.1:1 in group B. On histology severe inflammation was seen in 2(20%), cholestasis 3(30%) and AIH score 7 in 08 (80%) of group A while in group B severe inflammation was seen in 2 (6.7%), cholestasis 7(23.3%) and AIH score 7 in 21 (70%) cases. These histological differences were not statistically significant (p> 0.05). Stage 3 and 4 fibrosis was seen in 80% cases which was same in both the groups. (Table 1). Mean AST was 165.80±83.05 IU/L in group A which was significantly high as compared to 95.67±49.28 IU/L in group B (p 0.0158). After treatment with immunosuppression, response was compared between the two groups on the basis of liver enzymes. Mean AST was significantly reduced in both the groups when compared to before treatment (p=0.0040 and p=0.0386 respectively).
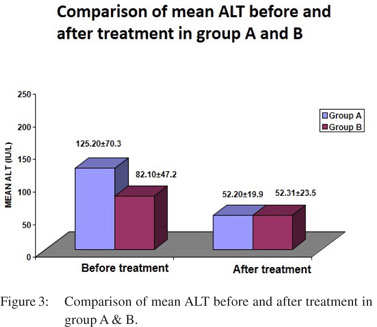
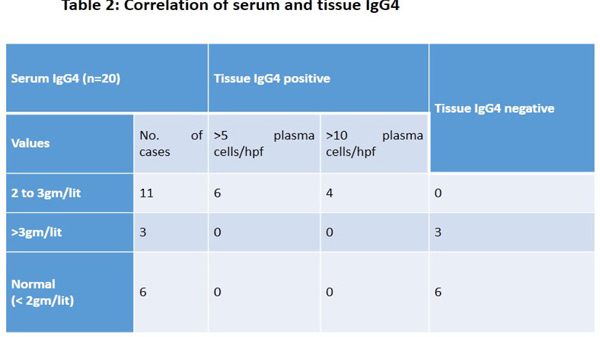
However the difference in two groups was not significant. (Figure 2). Before treatment mean ALT was higher in group A (125.20 ± 70.3 IU/L) than group B (82.10 ± 47.2 IU/L) but the difference was not statistically significant (0.0786). After the treatment, mean ALT reduced significantly (52.20±19.9 IU/L) in Group A (p=0.0093) as compared to group B however the difference was insignificant (p=0.0826). (Figure 3). Mean ALP before treatment was 272.40± 242.6 IU/L among Group A which was more as compared to 210.23 ±215.9 IU/L in Group B but the difference was insignificant. After the treatment, mean ALP did not show any significant change in both the groups. Mean total bilirubin before treatment was 5.27±4.6 mg/dL in Group A, which was comparable to 3.61±4.1 mg/dL among Group B. After the treatment, there was no significant change in total bilirubin in both the groups. Serum IgG4 was available in 20, raised levels between 2 to 3 gm/lit were seen in 11 cases. Only 3 cases showed serum IgG4 > 3 gm/lit and remaining 6 showed normal serum IgG4 levels. All 11 cases were tissue IgG4 plasma cells positive. Of these in 4 cases, IgG4 plasma cells were > 10/hpf and remaining showed > 5 / hpf. None of the cases with serum IgG4 > 3 gm/lit showed tissue positivity for IgG4 plasma cells. All serum negative cases were also negative for tissue IgG4 plasma cells (Table 2).
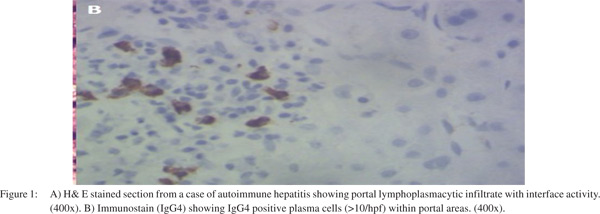

Discussion
Chronic inflammatory infiltrate rich in plasma cells is a feature of autoimmune hepatitis. Immunoglobulin IgG is produced by plasma cells in cases of autoimmune hepatitis. Some of these patients show increased subclass of immunoglobulin IgG4 which has been labelled as IgG4 related autoimmune hepatitis. There is limited data of IgG4 autoimmune hepatitis in the literature with ambiguous results. [20-23,26,27] Umemura T et al20 in 2007 first reported a case of type 1 AIH with cholecystitis showing raised serum IgG4 and liver histology consisting of abundant IgG4 plasma cells without pancreatic involvement. In 2009 Chung et al[22] reported 26 patients of autoimmune hepatitis, 10 primary biliary cirrhosis, 3 primary sclerosing cholangitis and 20 chronic hepatitis with HCV infection. Immunoreactivity for IgG4 plasma cells (> 5 / hpf) were 26 (34.6%) in AIH. None of the PBC, PSC and HCV cases showed positivity. On comparing IgG4 plasma cells positive and negative cases, there was neither significant increase in serum IgG4 levels nor liver enzymes between the two groups. These results are similar to our study in which IgG4 plasma cells were found to be 25 % in AIH when criteria of > 5 IgG4 plasma cells were considered. While only 4 cases (10%) showed > 10 IgG4 plasma cells positivity in this study. In contrast, Umemura T et al[21] in 2011 investigated serum and tissue IgG4 in liver biopsies of 60 Japanese patients with type 1 AIH and 22 autoimmune pancreatitis. Strict criteria were applied, such as definite diagnosis of AIH using scoring system, Serum IgG4 135 mg/ dL, IgG4 plasma cells 10/HPF and ratio of IgG4 to IgG > 0.073. Using these criteria prevalence of IgG4 plasma cells in AIH was 3.3% (2 of 60 cases) which was significantly lower when compared to patients with autoimmune pancreatitis. It has been shown that serum IgG4 value correlates with tissue IgG4 bearing plasma cells. Limitation of this study was smaller sample size hence we could not establish correlation of serum IgG4 with tissue IgG4 plasma cells. Serum IgG4 > 135 mg/dL has been shown to have sensitivity 97% and specificity 79.3% for diagnosis of IgG4 disease.[28,29] However since IgG4 is considered a systemic disease, raised serum IgG4 has been reported in various non pancreatobiliary conditions.[10] A variety of inflammatory conditions can show high levels of serum IgG4, leading to poor specificity and low predictive value. For diagnosis of this condition along with serum or tissue IgG4, clinicopathological correlation plays an important role. Liver enzymes and severity of inflammation on histology in group A was significantly higher in this study. It is a well known fact that the degree of IgG4 plasma cells correlates with the severity of disease and also the fibrosis.[23,27] However there was no difference in the stage of fibrosis between IgG4 positive and negative case.
Cholestasis is not an usual feature of AIH. From 40 cases of AIH, moderate to severe cholestasis was seen in 6 of which 3 showed > 10 IgG4 plasma cells/hpf, of these two patients died of liver disease. Meticulous history taking did not reveal use of drugs or toxins. Biliary pathology was ruled out on radiological examination. There are reports in literature on cholestatic presentation of AIH. These patients have higher chance of treatment failure and mortality.[30-32] Chung et al[22] have shown significantly reduced serum ALT levels in patients with IgG4 AIH after treatment. Reduced levels were maintained at 48, 72 and 96 weeks after initiating prednisolone therapy. In this study at the end of 4 weeks AST and ALT levels significantly reduced in group A as compared to group B but the difference between the 2 groups was not statistically significant. All patients were further followed up for 2 years and showed good response to treatment. There was no significant difference in treatment response between IgG4 positive and negative cases. In conclusion, IgG4 positive AIH patients were younger with more abnormal liver enzymes.
There was no difference in histology and response to the treatment in both groups. Larger population-based studies are required with long term follow up of these patients to determine response to immunosuppression.
References
- Czaja AJ. Autoimmune hepatitis in diverse ethnic populations and geographical regions. Expert Rev Gastroenterol Hepatol. 2013;7:365–85.
- Wong RJ, Gish R, Frederick T, Bzowej N, Frenette C. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol. 2012;46:155–61.
- Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–47.
- Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J Hepatol. 2000;32:181–97.
- Gupta R, Agarwal SR, Jain M, Malhotra V, Sarin SK. Autoimmune hepatitis in the Indian subcontinent: 7 years experience. J Gastroenterol Hepatol. 2001;16:1144–8.
- Amarapurkar DN, Amarapurkar AD. Role of autoimmunity in nonviral chronic liver disease. J Assoc Physicians India. 2000;48:1064–9.
- Amarapurkar DN, Patel ND. Spectrum of autoimmune liver diseases in western India. J Gastroenterol Hepatol. 2007;22:2112–7.
- Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76.
- Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824–32.
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51.
- Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77.
- Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23:57–66.
- Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4- Related Disease. Annu Rev Pathol. 2014;9:315–47.
- Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K,et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–4.
- Shinagare S, Shinagare AB, Deshpande V. Autoimmune pancreatitis: a guide for the histopathologist. Semin Diagn Pathol. 2012;29:197–204.
- Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172–9.
- Ketwaroo GA, Sheth S. Autoimmune Pancreatitis. Gastroenterol Rep. 2013;1:27–32.
- Kawa S, Hamano H, Umemura T, Kiyosawa K, Uehara T. Sclerosing cholangitis associated with autoimmune pancreatitis. Hepatol Res. 2007;37:S487–95.
- Nishimori I, Otsuki M. Autoimmune pancreatitis and IgG4- associated sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2009;23:11–23.
- Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–2.
- Umemura T, Zen Y, Hamano H, Joshita S, Ichijo T, Yoshizawa K, et al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol. 2011;46:48–55.
- Chung H, Watanabe T, Kudo M, Maenishi O, Wakatsuki Y, Chiba T. Identification and characterization of IgG4-associated autoimmune hepatitis. Liver Int. 2010;30:222–31.
- Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–71.
- Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–17.
- Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4- positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23–8.
- Koyabu M, Uchida K, Miyoshi H, Sakaguchi Y, Fukui T, Ikeda H, et al Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010;45:732–41.
- Yada N, Kudo M, Chung H, Watanabe T. Autoimmune hepatitis and immunoglobulin g4-associated autoimmune hepatitis. Dig Dis. 2013;31:415–20.
- Masaki Y, Kurose N, Yamamoto M, Takahashi H, Saeki T, Azumi A, et al. Cutoff Values of Serum IgG4 and Histopathological IgG4+ Plasma Cells for Diagnosis of Patients with IgG4-Related Disease. Int J Rheumatol. 2012;2012:1–5.
- Ohara H, Nakazawa T, Kawa S, Kamisawa T, Shimosegawa T, Uchida K, et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol. 2013;28:1247–51.
- Wang SB, Wang JH, Zheng RH, Zhong BH, Chen MH. Deep cholestatic jaundice as the predominant manifestation in autoimmune hepatitis. Hepatogastroenterology.2010;57:326–9.
- Tang CP, Shiau YT, Huang YH, Tsay SH, Huo TI, Wu JC, et al. Cholestatic jaundice as the predominant presentation in a patient with autoimmune hepatitis. J Chin Med Assoc. 2008;71:45–8.
- Yeoman AD, Westbrook RH, Zen Y, Maninchedda P, Portmann BC, Devlin J, et al. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology. 2011;53:926–34.
|
|
|
 |
|
|