|
|
|
|
 |
 |
| |
 |
|
|
Case Report |
|
|
|
|
|
Keywords :
|
|
|
Priyanka Saxena1, Chhagan Bihari1, Archana Rastogi2, Shiv Kumar Sarin3
Department of Hematopathology1,
Hepatopathlogy2, Hepatology3
Institute of Liver and Biliary Sciences
D-1, Vasant Kunj,
New Delhi-110070, India
Corresponding Author:
Dr. Priyanka Saxena
Email: docpriya06@rediffmail.com
DOI:
http://dx.doi.org/10.7869/tg.268
48uep6bbphidvals|728 48uep6bbph|2000F98CTab_Articles|Fulltext Non-cirrhotic portal fibrosis (NCPF) and extra-hepatic portal venous obstruction (EHPVO) are important causes of noncirrhotic PHT. The most common cause in EHPVO patients leading to PHT is portal vein thrombosis (PVT). Various mechanisms for PVT have been identified like portal vein injury, congenital defects, hypercoagulable states etc.[1]
Hypercoagulable states may be due to inherited gene mutations[2] or acquired causes. Acquired causes include use of oral contraceptives, pregnancy and myeloproliferative disorders(MPDs). Both latent and occult MPDs are known to constitute an important cause of PVT.[3]
We report the case of a 30-year old male patient with EHPVO who underwent successful shunt surgery and splenectomy with sudden precipitation of myeloproliferative disorder (MPD), post splenectomy.
Case presentation
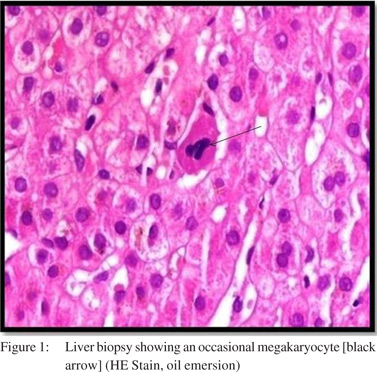
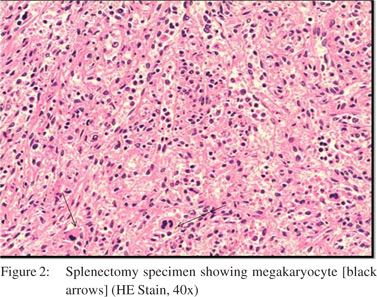
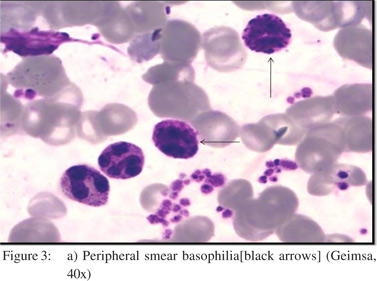
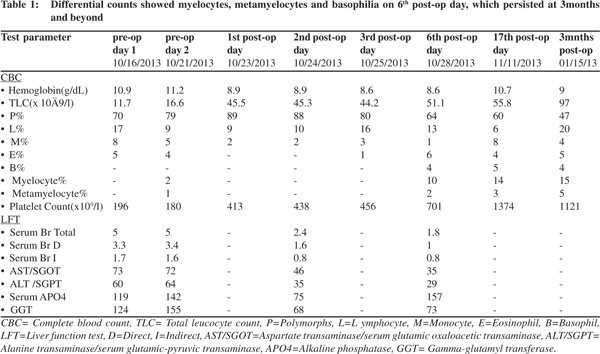
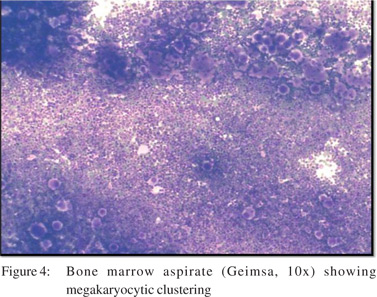
A30-year old male patient presented to the outpatient department of our institute in October 2013 with complaints of generalized weakness, pain in right upper quadrant, jaundice with high colored urine, pruritis, and intermittent clay-colored stools; all symptoms spanning a four-month period prior to presentation.On examination the patient had icterus with hepatosplenomegaly. Vitals were within normal limits.Patient had a significant past history.He was a known case of EHPVO diagnosed in 2008, non-bleeder (status EVL [endoscopic variceal ligation] done in 2010) with PHT.Upper GI endoscopyshowed grade 2 esophageal varices. CECT revealed chronic portal vein thrombosis with multiple porto-systemic collaterals and splenomegaly.MRI (abdomen) done in July2013 reported EHPVO with portal cavernoma , CBD compression and IHBRD, consistent with portal biliopathy.Liverbiopsy also carried out in November 2013 showed chronic obstructive biliary pathology with portal fibrosis (consistent with portal biliopathy) and an occasional focus of extramedullaryhematopoiesis (EMH) (Figure 1).Complete blood counts[CBC]were within normal limits.Liver Function tests[LFTs] were derangedand kidneyfunction testswere within normal limits.On the basis of clinical history, past history, examination and investigations the patient was diagnosed as:”EHPVO with PHT, asymptomatic hypersplenism, symptomatic portal biliopathy and surgical obstructive jaundice.”In view of the above diagnosis, the patient was planned for splenectomy with proximal splenorenal shunt. In Oct 2013, the patient underwent successful shunt surgery Specimen pathology revealedfibrocongestive spleen with occasional foci of EMH (Figure 2). LFT showed improvement (Table 1). CBC showed an increase in platelet counts and total leucocyte counts(TLC). The platelet counts increased from 180 x 109/L (pre-op) to 701 x 109 /L (6th post –op day) to 1121 x 109 /L (3months post-op). The TLC increasedfrom 16.6 x 109 /L (pre-op) to 51.1 x 109 /L (6th post –op day) to 97.0 x 109 /L ( 3months post-op). The differential counts showed myelocytes, metamyelocytes and basophilia on 6th post-op day, which persisted at 3months and beyond (Table 1). Peripheral smear showed absolute neutrophilia with shift to left, absolute basophilia and thrombocytosis (Figure 3). RBCs showed presence of target cells and Howell Jolly bodies. In view of the asymptomatic progressive neutrophilia with shift to left, basophilia and thrombocytosis a diagnosis of Chronic Myeloid Leukaemia(CML)was considered and subsequently bone marrow aspiration, biopsy and cytogenetic studies were carried out.Bone marrow aspirate revealedhypercellular marrow with myeloid and megakaryocytic hyperplasia with M:E ratio of 4:1 (normal 2-3:1) (Figure 4). Bone marrow biopsy showed hypercellular marrow with loss of fat spaces, myeloid and megakaryocytic hyperplasia and increased myeloid precursors. The reticulin stain did not show any fibrosis. JAK2V617F mutation for MPDs was negative. Flouresence in-situ hybridisation (FISH) for BCR-ABL (Philadelphia) translocation showed thespecimen to be positive for t(9;22) (q34;q11.2).On the basis of above investigations patient was diagnosed as a case of Philadelphia positive CML, chronic phase.
The development of thrombocytosis resulted in failure of the spleno-renal shunt. A follow up CT reported features suggestive of EHPVO with portal biliopathy and PHT. Splenic vein and spleno-renal shunt were not visualised most likely due to thrombosis. Patient was given double anti-platelet drugs and referred to hematological center for treatment of CML.
Discussion
We presented the case of a 30-year old young male with EHPVO, PHT, splenomegaly and symptomatic biliopathywho underwent splenectomy and successful shunt surgery. Afew points in this case required explanation and are subsequently discussed: Presence of leucocytosis and thrombocytosis post splenectomy. Is it normal or abnormal?
Our case had leucocytosis and thrombocytosis post splenectomy. Several studies have reported the presence of post-op neutrophilia and thrombocytosis. Shallaly et al have noted reactive thrombocytosis in patients with splenectomy, which increased to extreme levels by the 21st post-op day but subsided subsequently, reaching normal levels by the 17th postop week. They have also reported reactive leucocytosis which subsided to normal levels by the 2nd post-op week.[4] Hirsh and Dacie have reported transient thrombocytosis post splenectomy. McBride et al have noted marked increase in total leucocyte counts in splenectomy patients.[5] Therefore it is normal to have transient rise of platelet counts and leucocyte counts post-splenectomy.
Did the patient have latent myeloproliferativedisorder which led to the development of EHPVO and subsequent biliopathy?
Our case demonstrated certain abnormal trends in post op period. No decline was noted in platelet count even after 10-12 weeks post-splenectomy. The counts continued to rise steadily. There was progressive absolute neutrophilia with shifted towards the left in the absence of infection. Additionally, our patient had post-op persistent basophilia which never subsided. All these findings led to the suspicion of development of MPD in the patient. Hence it appears that our case had an underlying “latent” MPD which precipitated following splenectomy. Several studies have shown that not only overt MPDs but also latent forms of the disease may lead to the development of PVT . According to Sarin et al
approximately 58% of EHPVO patients in the western population had latent MPD.[6] Valla et al[3] and Primignani et al[7] have shown that latent MPDs are an important cause of portal vein thrombosis. Primignani has even advocated the use of JAK2 V617F molecular marker to confirm MPD in EHPVO patients.
Hence, from the above discussion it is apparent that our case had a latent MPD,which led to the development of EHPVO. The occurrence of EMH in spleenand liver. Several studies have reported the occurrence of splenic EMH in MPDs, thereby leading to splenomegaly. Sakakura et al have attributed the enlargement of liver and spleen in CML to EMH.8 Few other authors have noted and reviewed the occurrence of EMH in MPDs.[9]
What caused the precipitation of the latent CML in this case?
Does splenectomyunmask latent MPDs or does CML develop thusduring its natural course, noticed due to the patient ongoing treatment? A thorough and dedicated literature search on this issue did not reveal any evidence of splenectomy being the cause of development of overt MPDs. Jansen and Leebeek have made important observations regarding the MPD being an etiological factor in EHPVO patients. They state that the occurrence of splenomegaly in these patients may be due to MPD rather than PHT. Additionally, they speculate that the platelets and leucocytes may be increased in the marrow of such patients, but the peripheral counts are normal due to hypersplenism.[10] Based on these observations we have tried to hypothesize the sudden precipitation of CML, postsplenectomy in our case.
Our Hypothesis:
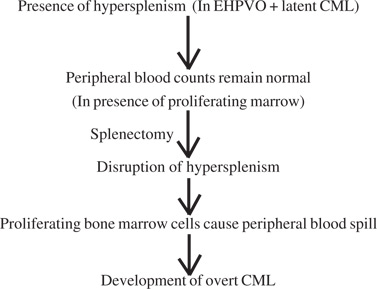
In conclusion, latent MPDs are an important cause of EHPVO and should be kept in mind whilst dealing with such patients. Patients with normal CBC in the presence of hypersplenism should be evaluated for MPD. Post splenectomy platelet counts should be closely monitored even after discharge and antiplatelet therapy continued till counts normalise. In cases of post splenectomybasophilia and absolute neutrophilia, the patient should be kept under close follow– up.
References
- Sarin SK, Agarwal SR. Extrahepatic portal venous obstruction. Semin Liver Dis. 2002;22:43–58.
- Denninger M H, Helley D, Valla D, Guillin MC. Prospective evaluation of the prevalence of factor V Leiden mutation in portal or hepatic vein thrombosis. ThrombHaemost. 1997;78:1297–8.
- Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, et al. Myeloproliferative disorders in portal vein thrombosis in adults. Gastroenterology. 1988;94:1063–9.
- Shallaly G, Karrar HK, Doumi A. Blood changes after splenectomy in portal hypertension. The ‘Amna Model’. Sudan JMS. 2013;8:47–56.
- Mc Bride JA, DacieV, Shapley R. The effect of splenectomy on leucocyte count. Brit J Haemat. 1968;14:225–31.
- Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, et al. Consensus on extra-hepatic portal veinobstruction. Liver International. 2006;26:512–9.
- Primignani M, Barosi G, Bergamaschi G, Gianelli U, Fabris F, Reati R, et al. Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology. 2006;44:1528–34.
- SakakuraM ,Ohishi K, Nomura K, Katayama N, Nishii K, Masuya M, et al. Case of chronic-phase chronic myelogenous leukemia with an abdominal hematopoietic tumor of leukemicclone origin. Am J Hematol. 2004;77:167–70.
- Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med. 2010;1:13–9.
- Janssen H, Leebeek F. JAK2 mutation: The best diagnostic tool for myeloproliferative disease in splanchnic vein thrombosis? Hepatology. 2006;46:1391–3.
|
|
|
 |
|
|