|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
non-celiac gluten sensitivity, irritable bowel syndrome, celiac disease |
|
|
Munish Ashat, Rakesh Kochhar
Department of Gastroenterology,
Post Graduate Institute of Medical
Education and Research,
Sector-12, Chandigarh - 160012,
India
Corresponding Author:
Dr. Rakesh Kochhar
Email: dr_kochhar@hotmail.com
DOI:
http://dx.doi.org/10.7869/tg.184
Abstract
There has been an increasing interest in non-celiac gluten sensitivity (NCGS) in recent years. The condition is characterized by both gastrointestinal and extra-intestinal symptoms that respond to gluten withdrawal. Most of the symptoms are subjective and for many years such patients remain in a diagnostic dilemma. Although symptomalogy is similar to irritable bowel syndrome (IBS), NCGS is now regarded as a distinct clinical entity. However, the disease pathology is not well elucidated and our knowledge of NCGS is still very rudimentary. This review highlights the importance of this new clinical entity, outlines its pathological mechanisms and suggests a diagnostic algorithm for its management.
|
48uep6bbphidvals|643 48uep6bbph|2000F98CTab_Articles|Fulltext Until recently the term gluten sensitivity included only celiac disease (CD) and wheat allergy (WA). Individuals with CD were recognised using serological markers like raised anti-tissue tranglutaminase (IgA tTg) levels or endomysial antibodies (anti-EMA) which have a positive predictive value of more than
90%.[1,2] The diagnosis is confirmed by demonstrating villous atrophy on duodenal biopsy. Similarly, WA is defined as an adverse immunologic reaction to wheat proteins which can clinically manifest as either classic food allergy affecting the skin, gastrointestinal tract or respiratory tract, wheatdependent, exercise-induced anaphylaxis (WDEIA), occupational asthma (baker’s asthma) and rhinitis, and contact urticaria. IgE antibodies play a central role in the pathogenesis of these diseases. Diagnosis is made by skin prick test and invitro IgE assays.[3] However, the positive predictive value of these tests is less than 75%, particularly in adults, due to their cross-reactivity with grass pollen. Further, non-IgE-mediated WA does exist and this form may be difficult to distinguish from gluten sensitivity.[4] However, there are subsets of patients, who are relieved of gastrointestinal symptoms on a gluten free diet and yet they do not fall under CD or WA. This observation has been long ignored but is recently gaining importance.
Definition and clinical spectrum
There is no well established definition for NCGS, formerly known as gluten sensitivity (GS). The London Consensus describes it as an entity distinct from CD and characterized by the lack of anti-tTG autoantibodies and any autoimmune comorbidities, with normal small intestine histology and resolution of symptoms with gluten free diet (GFD).[4] However, the two conditions cannot be distinguished clinically, since the symptoms experienced by GS patients are often seen in CD. Majority of symptoms experienced by these patients are subjective including abdominal pain, headaches, brain fog, tingling and/or numbness in hands and feet, fatigue and musculoskeletal pain. However, sometimes patients also develop diarrhea, rash, and severe neurological symptoms like schizophrenia and cerebellar ataxia.[5-7] But psychiatric manifestations lack strong evidence. Since most of the time patients had only subjective symptoms, they were frequently referred to a psychiatrist and remained a diagnostic dilemma.
Evidence in favour of NCGS
Non-celiac gluten sensitivity was first described in 1978 with a case report published in Lancet describing a patient with diarrhea and intermittent abdominal pain without any abnormality on intestinal biopsy and who was relieved of symptoms when placed on GFD.[8] This was followed by a small pilot study by Cooper et al in 1980 with eight female subjects who had abdominal pain and chronic diarrhea without any serological or histological evidence of CD. When placed on GFD they had a dramatic relief of symptoms and when they were given gluten re-challenge they had immediate return of symptoms. Jejunal biopsy from these patients showed significantly increased cellularity which returned to normal after reintroducing GFD.[9]
More recently Kaukinem et al also demonstrated that gluten intolerance is not specific to celiac disease or wheat allergy only. Out of 93 patients enrolled in their study with abdominal symptoms on gluten ingestion only eight had CD. Seven patients were diagnosed as latent CD and 19 had a positive allergy test. Rest 61 patients who benefitted from GFD were regarded as affected from NCGS.[10] Similar results were reported by Campanella et al, who recruited 112 patients out of 180 patients referred to them, who had been diagnosed with CD based on improper diagnostic criteria. Subsequent duodenal biopsies and endomysial antibodies confirmed the diagnosis of CD in only 51 patients. A gluten free diet improved the symptoms in 64.7% CD patients and 75% non-celiac disease patients. Re-introduction of gluten resulted in clinical exacerbation in 71.4% of celiac disease patients and 54.2% of non-celiac disease patients. Although researchers concluded that clinical response to either withdrawal or re-introduction of dietary gluten has no role in the diagnosis of CD, but it also pointed out the existence of another form of gluten sensitivity.[11]
Thus these studies have laid down the evidence for NCGS as a distinct entity. However, the placebo effect of gluten ingestion in self-diagnosed gluten sensitivity cases cannot be ruled out and has been clearly demonstrated in double-blind studies.[12-14]
There has been a recent increase in perception among the general public that gluten is harmful. Gluten which is the main protein in wheat, barley and rye has been a relatively recent introduction to human diet. Humans have existed for 2.5 million years and grains were first introduced in our diet only 10,000 years back providing circumstantial evidence that humans might not be well adapted for diet rich in gluten. The two main constituents of gluten protein are gliadins and glutenins which are resistant to gastric digestion and increase intestinal permeability causing malabsorption and inhibition of cell growth and apoptosis.[15] These features are consistent with CD, a well know gluten sensitivity disorder. Media and general public interest in this topic is overwhelming. This is evident by the number of Google to Pubmed hits for GFD (~5000:1).
Although, NCGS is still a controversial topic, but nonetheless, wide spread media hype and celebrity endorsements have made GFD a new diet craze among the general public. A US market survey has shown a rapid increase in sales of GFD which is expected to touch $ 4.3 billion by 2015.[16] Dietary trends are changing with low carbohydrate diet which had widespread acceptance in the late 20th century, gradually declining over time and now has been replaced by GFD as a major dietary trend.[17]
Although the prevalence of GS disorder is around 6% but the GFD is purchased by 17-25% of the US population.[16] Increased awareness and knowledge about GS can only explain a fraction of these sales. Another issue that needs clarification is whether gluten or some other component of wheat is responsible for the gastrointestinal (GI) symptoms. Gluten as an independent cause of GI symptoms has never been directly assessed. Wheat also contains other proteins, carbohydrates and lipids which can also be responsible for the GI symptoms.
Pathophysiology
Understanding the pathophysiology of NCGS is important but unlike CD, where the pathology is well established, NCGS has many grey areas (Table 1). In order to understand the pathophysiology of NCGS we first need to understand the pathogenesis of CD and how these two clinically similar conditions differ at cellular level. Gluten is not completely digested by stomach enzymes. Gliadin induces apoptosis and alters permeability in in-vitro models[18] and is also believed to increase intestinal distension due to intestinal fermentation of poorly absorbed gluten, although no evidence has been found in support of this hypothesis.[19] In CD impaired epithelial function allows these peptides to enter the lamina propria where they are recognised by tissue transglutaminase type 2 (tTG2) and deamidated. These deamidated peptides have increased negative charge on them which increases their binding to HLADQ2/ 8 receptors present on antigen presenting cells.[20] A vast majority of CD cases have HLA-DQ2/8 allele variants.
Although, there are seven different types of HLA-DQ variants but only these two variants have been shown to bind strongly enough to gluten epitopes to initiate a CD4+ T cell activation process. Upon activation, the CD4+ T cells produce proinflammatory cytokines like tumour necrosis factor-á, interferon-Ò, interleukin (IL)-15, IL-10, IL-6, IL-21 and IL-17.[21] IL-15 which is considered to be the first trigger in CD development, is also released in healthy individuals upon gluten ingestion, but does not reach significant levels to cause inflammatory reaction in the gut.[22] This difference is mainly attributed to increased IL-15 receptor expression in celiac patients.[22,23] Furthermore, activation of B lymphocytes by INFÒ produces anti-tTG antibodies which trigger adaptive immunity. Thus both adaptive and innate immunity play a role in development of CD.
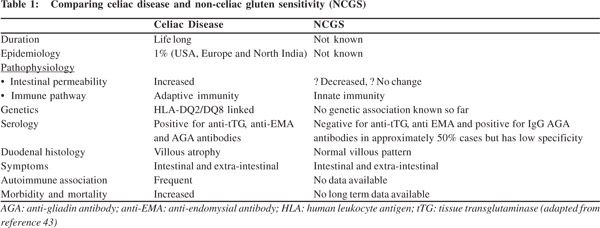 In contrast, toll-like receptor (TLR) expression, particularly TLR-2, which plays a key role in innate immunity, has been found much more upregulated in subjects with NCGS than more with CD.[24] Moreover, Sapone et al demonstrated adaptive immunity markers IL-6, IL-17, IL-21 and INF-Ò were overexpressed in CD but not in NCGS.[24] FOXP3, a T-regulatory marker which helps in maintaining immune homeostasis by limiting immune response, has been found significantly decreased in NCGS as compared to CD and healthy controls. This further indicates the role of innate immunity in NCGS.[25]
NCGS subjects also have increased number of á/â subtype intraepithelial lymphocytes (IEL) which are different from the Ò/ä subtype IELs found in celiac disease, substantiating the role of innate immunity in NCGS.[26] Another important aspect in which NCGS differs from CD is epithelial permeability. Sapone et al have demonstrated an increase in claudin-4 expression in NCGS, which is a marker of decreased epithelial function. Impaired epithelial function is further demonstrated by the lactulose/mannitol absorption test. Further, PCR analysis of tight junction components in duodenal biopsies of NCGS patients has shown increased CLDN4 mRNA levels, which is a marker of decreased intestinal permeability. All this evidence supports the hypothesis that NCGS is marked by decreased intestinal permeability.[24] In contrast, claudin-1 and ZO-1 are seen elevated in CD with increased epithelial permeability.[27]
In contrast, toll-like receptor (TLR) expression, particularly TLR-2, which plays a key role in innate immunity, has been found much more upregulated in subjects with NCGS than more with CD.[24] Moreover, Sapone et al demonstrated adaptive immunity markers IL-6, IL-17, IL-21 and INF-Ò were overexpressed in CD but not in NCGS.[24] FOXP3, a T-regulatory marker which helps in maintaining immune homeostasis by limiting immune response, has been found significantly decreased in NCGS as compared to CD and healthy controls. This further indicates the role of innate immunity in NCGS.[25]
NCGS subjects also have increased number of á/â subtype intraepithelial lymphocytes (IEL) which are different from the Ò/ä subtype IELs found in celiac disease, substantiating the role of innate immunity in NCGS.[26] Another important aspect in which NCGS differs from CD is epithelial permeability. Sapone et al have demonstrated an increase in claudin-4 expression in NCGS, which is a marker of decreased epithelial function. Impaired epithelial function is further demonstrated by the lactulose/mannitol absorption test. Further, PCR analysis of tight junction components in duodenal biopsies of NCGS patients has shown increased CLDN4 mRNA levels, which is a marker of decreased intestinal permeability. All this evidence supports the hypothesis that NCGS is marked by decreased intestinal permeability.[24] In contrast, claudin-1 and ZO-1 are seen elevated in CD with increased epithelial permeability.[27]
But the data is not consistent as some studies have demonstrated higher epithelial permeability in HLA-DQ2/8+ NCGS subjects with irritable bowel syndrome, diarrhea and gluten sensitivity[24,28] while other found no alterations in epithelial permeability in treated NCGS subjects who were given gluten challenge versus those given placebo.[29]
Proteins other than gluten can also be responsible for the differences between NCGS and CD. Bucci et al demonstrated that basophils derived from gut mucosa of patients with NCGS were not activated by gliadin.[30] In a recent in-vitro study it was found that á-amylase/trypsin inhibitors (ATI), which are a group of low molecular weight proteins found in wheat, can act as pro-inflammatory agents causing the release of inflammatory cytokines from dendritic cells, monocytes and macrophages in both CD and NCGS patients. This is mediated by the interaction between ATI and the TLR4-MD2-CD14 complex.[31] Apart from various proteins, food rich in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) have also been implicated in NCGS. A randomised, double-blind, placebocontrolled, cross over trial by Biesiekirski et al demonstrated that NCGS patients put on diet low in FODMAP had reduced gastrointestinal symptoms. When these patients were reintroduced to gluten or whey protein their symptoms worsened.[32] However, NCGS patients are known to suffer from multiple food hypersensitivities and FODMAPs cannot be exclusively implicated for the disease. Moreover, many patients who are on GFD continue to consume other sources of FODMAP but still remain asymptomatic. Further double-blind studies are required to understand the triggers of NCGS.
Epidemiology
With improved and more sensitive serological testing there has been an increase in number of patients diagnosed with CD. Epidemiological studies indicate the prevalence in North America and Western Europe to be 0.5-1%.[33] The prevalence varies from country to country with 2% in Finland and 3% in Germany.[34] Experts agree that CD displays the iceberg phenomenon with far less cases being diagnosed than their actual prevalence in the general population. The prevalence has increased over past two decades and is estimated to be between 1 in 100 and 1 in 300.[35,36] Although HLA-DQ2 and DQ8 have been associated with CD, they are not the only markers as these HLA haplotypes confer only 30-40% genetic predisposition to CD.21 In the Asian population, CD is less prevalent in Indonesia, Philippines and South Korea. In India the prevalence varies from 1.23% (northern India) to 0.13% (southern India) (ICMR Task Force Report 2014). The genetic determinants for CD (HLA-DQ2 and HLA-DQ8) have been found in one-third of healthy north Indian population and oneeighth of south Indian population.[37,38] Thus both genetic and environmental factors (wheat is a staple diet in North India) possibly contribute to the higher prevalence of CD in north India.
In contrast very little is known about the prevalence of NCGS. Center for Celiac Research at the University of Maryland found 347 patients out of 5896 subjects, who satisfied the criteria for NCGS, translating into a prevalence of 6%. But the researchers concluded that this higher prevalence can be due to referral bias. Furthermore, the true prevalence is unknown because GFD has emerged as a new dietary trend.[13] Although NCGS can occur in any age group but adults are more prone than children, with a median age of onset of 40yrs (range: 17-63 yrs). NCGS is more prevalent in females (1:2.5) and in patients of IBS.[39] More epidemiological studies are required to delineate the actual prevalence of NCGS in general population. No studies have attempted to elucidate the prevalence of NCGS in Asians.
Overlap with IBS
IBS and NCGS have a complex relationship. Gluten withdrawal has been shown to benefit IBS-D patients. A randomised controlled 4-week trial at the Mayo Clinic compared gluten containing diet (GCD) and gluten free diet (GFD) in 45 IBS-D patients. The researchers found that IBS-D patients on GFD (n=23) had statistically significant relief in gastrointestinal symptoms compared to the GCD (n=22) arm. GCD had a greater effect on bowel movements, form and ease of passage; although the effect on stool frequency was greater in HLADQ2 or DQ8 positive patients than HLA-DQ2/8 negative patients. Moreover there was no significant decrease in intestinal permeability. However patients on GCD had a significant decrease in expression of zonula occludens 1, claudin-1 and occludin in rectosigmoid mucosa, which was also prominent with HLA-DQ2/8 positive genotype. There was no difference in food transit and histology among GCD and GFD group. This study demonstrated that IBS patients can benefit from GFD particularly in HLA-DQ2/8 positive patients.[29]
One of the first studies to link NCGS with IBS was carried out by Biesiekierski et al. The investigators undertook a doubleblind, randomised, placebo-controlled re-challenge trial in patients with IBS fulfilling the ROME III criteria. Patients who were on self-imposed GFD diet were enrolled through advertisement in e-newspaper and through referrals from private clinics. The aim was to test if gluten can cause gastrointestinal symptoms in patients without CD. After excluding all patients with CD by duodenal biopsy on a gluten diet or a negative HLA-DQ2/8 test, a total of 34 patients were enrolled in the study. After randomisation the patients were followed for six weeks on GFD, with one group of 19 patients receiving 16 g of carbohydrate depleted gluten per day in form of two bread slices and a muffin and another group of 15 patients received gluten-free muffins and two bread slices everyday for six weeks. Gluten was also tested for FODMAPS and was found to be free from short chain carbohydrates, fructans, fructose, glucose, sorbitol, mannitol, raffinose and kestose. Although the gluten group experienced a higher pain severity score and bloating than the placebo group, there was no evidence of increase in celiac disease biomarkers. Fecal lactoferrin, which is a marker of intestinal inflammation and intestinal permeability, was also found to be normal in NCGS patients even after gluten ingestion.[28] This landmark study not only proved the presence of NCGS but also the role of gluten in the development of symptoms.
However, IBS-like symptoms cannot be exclusively attributed to wheat. In a retrospective, double-blind, placebo controlled re-challenge study by Carroccio et al, patients with IBS were restricted from cow’s milk, wheat, eggs and chocolate for 4 weeks. This was followed by re-challenge with wheatfilled capsules. 920 patients diagnosed with IBS by the Rome-II criterion and fulfilling the criterion for gluten sensitivity were included in the trial. After 4 weeks of diet restriction and doubleblind wheat challenge only 276 patients (30%) became asymptomatic and their symptoms reappeared with gluten challenge. They were subjected to another 4 week diet restriction, followed by re-challenge with only cow’s milk protein. 70 patients remained asymptomatic on cow’s milk protein and were labelled as suffering from only NCGS, whereas 206 patients who redeveloped symptoms were classified as having multiple food allergies. This study not only suggests NCGS as a distinct entity which is prominent in IBS patients but also suggests two distinct types of population among NCGS: one with only wheat allergy and the other with multiple food allergies. But the authors highlighted that the intrinsic drawback of the study was that it was a retrospective study and it may be possible that some of the patients who were categorised as having NCGS were actually CD patients since their duodenal biopsy had mostly not been taken from the duodenal bulb, which has recently been recommended as a preferred site for CD diagnosis. Furthermore, the wheat capsule would have many other components other than gluten that could trigger GI symptoms in these patients. Of particular importance are FODMAPs which can alter the gut microbiota inducing IBS-like symptoms. In a placebo-controlled, crossover re-challenge study by Biesiekierski et al no evidence of dose-dependent effects of gluten was found, in patients with NCGS placed on diets low in FODMAPs.[32] This was in contrast to a double-blind, randomized, placebo-controlled re-challenge trial undertaken by the same group in patients with IBS in whom CD was excluded and who were symptomatically controlled on a GFD.[28] These contradictory results are difficult to interpret.
Serology and histology
Unlike CD there are no serological markers for detecting NCGS. In a retrospective study, 78 NCGS and 80 CD patients was compared for serological markers. IgG anti-gliadin antibody (AGA) was positive in 56.4% NCGS patients compared to 81.2% CD patients. IgA AGA was found in 7.7% NCGS patients and 75% CD patients. IgA tTG and IgA EMA were invariably negative in NCGS patients. Only one patient out of 78 NCGS patients was positive for IgG deamidated gliadin peptide antibodies (DGP-AGA). The study concluded that a majority of NCGS patients are positive for IgG AGA and IgA tTG and IgA EMA are always negative in NCGS unlike CD.39 HLA-DQ2 or HLA-DQ8 is positive in 95% of CD patients compared to only 50% NCGS patients. Thus there is no specific biomarker for the diagnosis NCGS. Since a considerable number of NCGS patients have HLA-DQ2 and DQ8 positivity, a duodenal biopsy is a must to rule out latent CD. Most of the work that has been done on histological classification of NCGS has reported NCGS as either normal (Marsh 0) or characterized by infiltration of intraepithelial lymphocytes (IEL) (Marsh 1). Most researchers have reported less than 25 IELs per 100 epithelial cells in nearly two-third NCGS cases but always with a normal villous pattern.[4] Sapone et al4 however found more than 30 IELs per 100 enterocytes in their NCGS subjects. Further, IELs can be attributed to several other causes and are not a specific histological marker for NCGS.[40] Recently, Not et al found mucosal deposits of IgA anti-TG2 in the intestines of 15 out of 22 (68%) patients with NCGS. Although latent CD has not been excluded these patients had negative serum anti-TG2 and no intestinal abnormality.[41] Furthermore, Carroccio et al recently reported an increase in intraepithelial eosionophils in the colonic mucosa of 174/276 (63%) NCGS patients (>4 intraepithelial eosinophils/ high power field). The count of the duodenal intraepithelial eosinophils was significantly higher in NCGS patients than in IBS controls. But the researchers concluded that the high eosinophil count could be due to many confounding factors like chronic constipation food allergies. However, they also highlighted that increased intraepithelial eosinophil count can be used to guide elimination diet trial in such patients.[42]
Management
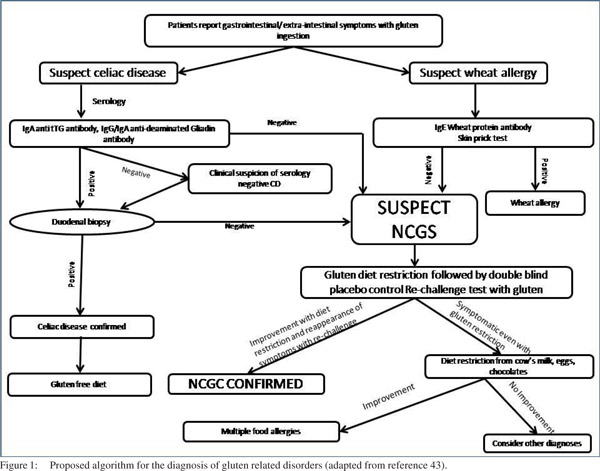
Most of the symptoms of NCGS are subjective. The clinical picture is characterized by GI (abdominal pain, bloating, diarrhea and/or constipation, nausea, epigastric pain, gastroesophageal reflux) and systemic manifestations (tiredness, headache, fibromyalgia-like joint/muscle pain, leg or arm numbness, ‘foggy mind,’ dermatitis or skin rash, depression, anxiety, and anemia). Due to lack of any diagnostic protocol or serological markers the diagnosis of NCGS remains clinical and is reached after exclusion of CD and wheat allergy by serology and duodenal biopsy. A double-blind placebo-controlled challenge should always be given in order to rule out placebo effect of wheat withdrawal and other food hypersensitivities. The authors suggest the following algorithm for diagnosis of NCGS (Figure 1).
Conclusion
NCGS is a distinct clinical entity which responds to gluten-free diet but has no serological or histological similarity to celiac disease. The link between IBS and NCGS has been the subject of recent research. Although wheat is responsible for most of the symptoms of NCGS, there is lack of evidence if gluten is the only gluten only trigger involved. Further double-blind, placebo controlled studies are needed to clarify this matter. A number of unanswered questions remain: how prevalent is NCGS in the general population, what is the underlying mechanism for NCGS, when should we suspect NCGS in a patient and should all patients with IBS be investigated for NCGS?
 References
References
- Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;128:S25–32.
- Volta U, Granito A, Parisi C, Fabbri A, Fiorini E, Piscaglia M, et al. Deamidated gliadin peptide antibodies as a routine test for celiac disease: a prospective analysis. J Clin Gastroenterol. 2010;44:186–90.
- Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009;9:238–43.
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
- Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med. 2011;269:582–90.
- Vlissides DN, Venulet A, Jenner FA. A double-blind gluten-free/ gluten-load controlled trial in a secure ward population. Br J Psychiatry. 1986;148:447–52.
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–4.
- Ellis A, Linaker BD. Non-coeliac gluten sensitivity? Lancet. 1978;1:1358–9.
- Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology. 1980;79:801–6.
- Kaukinen K, Turjanmaa K, Maki M, Partanen J, Venalainen R, Reunala T, et al. Intolerance to cereals is not specific for coeliac disease. Scand J Gastroenterol. 2000;35:942–6.
- Campanella J, Biagi F, Bianchi PI, Zanellati G, Marchese A, Corazza GR. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008;43:1311–4.
- Tanpowpong P, Broder-Fingert S, Katz AJ, Camargo CA, Jr. Predictors of gluten avoidance and implementation of a glutenfree diet in children and adolescents without confirmed celiac disease. J Pediatr. 2012;161:471–5.
- DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. Scand J Gastroenterol. 2013;48:921–5.
- Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1–4.
- Blomfeldt TO, Kuktaite R, Johansson E, Hedenqvist MS. Mechanical properties and network structure of wheat gluten foams. Biomacromolecules. 2011;12:1707–15.
- Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6:43–55.
- Makarem N, Scott M, Quatromoni P, Jacques P, Parekh N. Trends in dietary carbohydrate consumption from 1991 to 2008 in the Framingham Heart Study Offspring Cohort. Br J Nutr. 2014:1–14.
- Hadjivassiliou M, Williamson CA, Woodroofe N. The immunology of gluten sensitivity: beyond the gut. Trends Immunol. 2004;25:578–82.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–8.
- Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. 2013;13:294–302.
- Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol. 2011;29:493–525.
- Di Sabatino A, Ciccocioppo R, Cupelli F, Cinque B, Millimaggi D, Clarkson MM, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–77.
- Bernardo D, Garrote JA, Allegretti Y, Leon A, Gomez E, Bermejo- Martin JF, et al. Higher constitutive IL15R alpha expression and lower IL-15 response threshold in coeliac disease patients. Clin Exp Immunol. 2008;154:64–73.
- Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23.
- Vorobjova T, Uibo O, Heilman K, Rago T, Honkanen J, Vaarala O, et al. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand J Gastroenterol. 2009;44:422–30.
- Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842–50.
- Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195–205.
- Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14.
- Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–11 e3.
- Bucci C, Zingone F, Russo I, Morra I, Tortora R, Pogna N, et al. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin Gastroenterol Hepatol. 2013;11:1294–9 e1.
- Junker Y, Zeissig S, Kim SJ, Barisani D, Wieser H, Leffler DA, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–408.
- Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–8 e1–3.
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92.
- Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–95.
- Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease? Gastroenterology. 2005;128:S47–51.
- Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–25.
- Sood A, Midha V, Sood N, Kaushal V, Puri H. Increasing incidence of celiac disease in India. Am J Gastroenterol. 2001;96:2804–5.
- Yachha SK, Poddar U. Celiac disease in India. Indian J Gastroenterol. 2007;26:230–7.
- Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol. 2012;46:680–5.
- Aziz I, Evans KE, Hopper AD, Smillie DM, Sanders DS. A prospective study into the aetiology of lymphocytic duodenosis. Aliment Pharmacol Ther. 2010;32:1392–7.
- Not T, Ziberna F, Vatta S, Quaglia S, Martelossi S, Villanacci V, et al. Cryptic genetic gluten intolerance revealed by intestinal antitransglutaminase antibodies and response to gluten-free diet. Gut. 2011;60:1487–93.
- Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–906.
- Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. 2013;10:383–92.
|
|
|
 |
|
|