|
|
|
|
 |
 |
| |
 |
|
|
Case Report |
|
|
|
|
|
Keywords :
|
|
|
Niteen Kumar1, Shridhar Vasantrao Sasturkar1, Archana Rastogi2, Viniyendra Pamecha1
Departments of HPB Surgery,1 and Pathology,2
Institute of Liver and Biliary Sciences,
D1- Vasant Kunj, New Delhi, India, 110070
Corresponding Author:
Dr. Viniyendra Pamecha
Email: viniyendra@yahoo.co.uk
DOI:
http://dx.doi.org/10.7869/tg.196
48uep6bbphidvals|655 48uep6bbph|2000F98CTab_Articles|Fulltext A telengiectatic adenoma (TA) is a rare, benign tumor of the liver, with potential for malignant degeneration.[1] Although the term is employed to connote the dilated vessels seen on histology, clinical and morphologic features indicate it to be a variant of focal nodular hyperplasia (FNH).[2] Their resemblance to liver cell adenoma (LCA) stems from the lack of fibrous scars characteristic of FNH, blurring the distinction between these conditions. Molecular genetics further reveal its monoclonal nature, a feature consistent with neoplastic growth.[3] Studies have concluded that TA comprises of a spectrum of benign liver diseases varying from LCA to FNH, instead of being one distinct entity.[4]
Case report
A 32-year-old male presented to us with upper abdominal pain. The patient did not have any history of jaundice and was a non-alcoholic. A large lesion was detected in the right lobe of liver on ultrasound (US). His serum bilirubin (both total and direct), aspartate transaminase (AST) and alanine transaminase (ALT) levels were normal but alkaline phosphatase and gamma glutamyl transferase (GGT) were raised. Viral serology for HBV and HCV was negative. He was referred to us for further evaluation. On repeat ultrasound the SOL measured 14×15×15 cm in size, and had replaced the entire right lobe of liver. It demonstrated mixed echogenicity with interspersed anechoic areas. Rest of the liver was normal in echotexture with occasional tiny cystic lesions. There was no biliary dilatation. The main portal vein and its branches were patent. With a provisional diagnosis of liver hemangioma, nuclear imaging (RBC-labeled scan) was undertaken which showed large cold lesions in the right lobe of liver with no significant RBC pooling in the region up till 24 hrs.
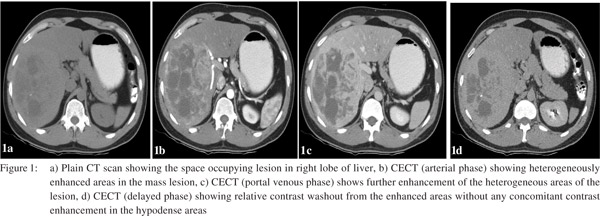
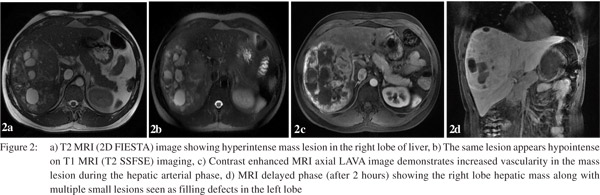
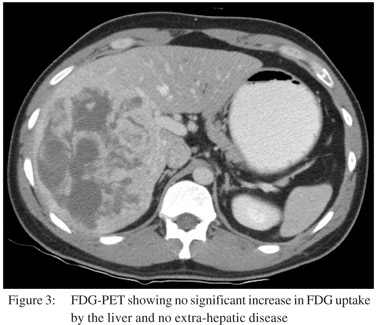
For further characterization a triple phase contrast enhanced CT (CECT) scan of the abdomen was performed. The right lobe lesion was occupying segments 5, 6, 7 and 8 and measured 15×11.5 cm in size. On plain scan some calcification were also seen in the lesion (Figure 1a) which were heterogeneously enhanced on arterial phase of CECT (Figure 1b). During the subsequent portal venous phase, enhancement in the lesion became relatively more prominent (Figure 1c). Hypodense areas in the lesion did not show significant enhancement neither in the arterial nor the delayed phase. Further, the enhanced areas in the arterial phase showed relative contrast washout during the delayed phase (Figure 1d), with no evidence of any centripetal contrast filling in the hypodense areas. Multiple small simple cysts were also seen in both lobes of the liver. The lesion displaced and appeared to encase the right hepatic vein and the anterior and posterior branches of the right portal vein; however, no thrombi were seen in the vessels. Branches of the right hepatic artery were seen coursing through the lesion. There was no lymphadenopathy or ascites. CT findings narrowed down the differential diagnoses to LCA or hepatocellular carcinoma (HCC). As the alpha-fetoprotein (AFP) level was within normal limits and venous phase wash out was absent, further imaging with contrast enhanced MRI (CEMRI) was done. The lesion showed heterogeneous signal intensity on T1 and T2 MRI images. It was predominantly hyperintense on T2 (Figure 2a) and hypointense on T1 (Figure 2b). On contrast administration the lesion showed increased hypervascularity during the hepatic arterial phase (Figure 2c) with central areas of non-uptake which were seen better during the portal venous and equilibrium phases. In addition multiple small lesions of varying size were seen in the left lobe appearing as filling defects during the portal venous and equilibrium phases (Figure 2d). The imaging revealed no evidence of cirrhosis. Further, USG guided needle aspiration remained inconclusive and did not add to the diagnostic workup. Finally FDG-PET showed no significant increased in FDG uptake by the liver and no evidence of any extra-hepatic disease (Figure 3).
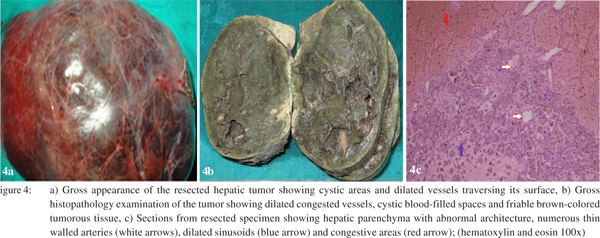
 In view of the uncertain nature and large size of the lesion and pain abdomen, we planned to perform liver resection. A right lobe hepatectomy via a plane of transaction lateral to the middle hepatic vein (MHV) was performed. Intraoperatively, there was no evidence of ascites, peritoneal metastases or cirrhosis. The mass was found involving almost the entire right half of liver. It had cystic areas and dilated vessels coursing its surface (Figure 4a). The portal vein, common bile duct and hepatic artery were normal with no direct involvement by the tumor. Multiple simple cysts were also seen on the left lobe. On subsequent histopathology the outer surface of the resected mass was well-encapsulated and showed few dilated congested vessels. On serial sectioning (Figure 4b) through the parenchyma, an irregular 16.5×15×6 cm mass with cystic blood filled spaces was found. The tumor was fragile and brown colored. The liver transection margin was free of tumor involvement and no capsular breach was seen. No central scar was seen on serial sectioning. On microscopic examination (Figure 4c) the tumor showed 1-2 cell thick cords of hepatocytes demarcated in places by thick fibrocollagenous bands. Prominent areas of congestion, peliosis and sinusoidal dilatation were also seen in the tumor. In a few areas, blood vessels and remnants of portal areas devoid of bile ducts were noted. Few small interspersed groups of inflammatory cells were also seen. The hepatocytes showed prominent cellular cholestasis, mild steatosis and mild nuclear pleomorphism in places. No evidence of malignancy was found in examined sections. The patient’s postoperative recovery was uneventful with a peak serum bilirubin of 2.9 mg/dl on third postoperative day. He was discharged after 8 days of surgery. He is asymptomatic with normal liver function and no evidence of recurrent disease after one year of follow-up.
In view of the uncertain nature and large size of the lesion and pain abdomen, we planned to perform liver resection. A right lobe hepatectomy via a plane of transaction lateral to the middle hepatic vein (MHV) was performed. Intraoperatively, there was no evidence of ascites, peritoneal metastases or cirrhosis. The mass was found involving almost the entire right half of liver. It had cystic areas and dilated vessels coursing its surface (Figure 4a). The portal vein, common bile duct and hepatic artery were normal with no direct involvement by the tumor. Multiple simple cysts were also seen on the left lobe. On subsequent histopathology the outer surface of the resected mass was well-encapsulated and showed few dilated congested vessels. On serial sectioning (Figure 4b) through the parenchyma, an irregular 16.5×15×6 cm mass with cystic blood filled spaces was found. The tumor was fragile and brown colored. The liver transection margin was free of tumor involvement and no capsular breach was seen. No central scar was seen on serial sectioning. On microscopic examination (Figure 4c) the tumor showed 1-2 cell thick cords of hepatocytes demarcated in places by thick fibrocollagenous bands. Prominent areas of congestion, peliosis and sinusoidal dilatation were also seen in the tumor. In a few areas, blood vessels and remnants of portal areas devoid of bile ducts were noted. Few small interspersed groups of inflammatory cells were also seen. The hepatocytes showed prominent cellular cholestasis, mild steatosis and mild nuclear pleomorphism in places. No evidence of malignancy was found in examined sections. The patient’s postoperative recovery was uneventful with a peak serum bilirubin of 2.9 mg/dl on third postoperative day. He was discharged after 8 days of surgery. He is asymptomatic with normal liver function and no evidence of recurrent disease after one year of follow-up.
 Discussion
Benign lesions of the liver like LCA are rare and can pose significant diagnostic challenge especially when they present uncommon variants.[5] Differentiation between FNH and LCA can be difficult. In the presence of a variant like TA, radiology findings may not be characteristic. Even needle biopsies can be unreliable and difficult to obtain, but they are an important part of investigations in such cases. TA resembles FNH due to its ductular reaction and nodular architecture; however lack of central scar, sinusoidal dilatation, naked arteries and frequent intralesional hemorrhages make it morphologically closer to LCA.[4] It appears that FNH and LCA comprise poles of a histological spectrum which ranges from fibrotic scar, arterioles and ductules in classic FNH to lack of fibrosis, naked arterioles and absence of ductular reaction in classic LCA, with TA lying somewhere between the two.[4]
LCA is overwhelmingly common in young women (92%) taking oral contraceptives and long duration of intake probably predisposes them to TA.2 This association however is not synonymous and LCA should not be construed as the precursor of TA, biological causality between the two being unclear. Other risk factors reported include obesity and inflammatory syndromes.[6] FNH is recognized with relative ease on imaging and once diagnosed requires no further treatment. Recently characterized radiological features of TA consists of a heterogeneous lesion with hyperintensity in T1-weighted MR images, strong hyperintensity in T2-weighted MR images, and persistent contrast enhancement in delayed-phase contrast enhanced CT or T1-weighted MRI with lack of central scar.[7] These findings were found to correlate well with histopathology features of TA.[5]
Much effort has been put to delineate the exact nature of this lesion. Molecular genetics of the neoplasm showed a much higher rate of monoclonality in TA compared to classic FNH. Although, the polyclonal nature of FNH argues against its neoplastic nature, as many as 50% tumors show X-chromosome inactivation suggestive of monoclonality, thus favoring a neoplastic nature.[8] Despite the demonstration of sinusoidal dilatation on histopathology and multiple tortuous, ectatic intralesional vessels on angiography, 99mTc labeled RBC studies often fails to detect any abnormality in TA. A probable explanation of this paradox is that although individual hepatic arteries in the lesion are enlarged and ectatic, they remain too small to be picked up by the 99mTc labeled RBC blood-pool technique. Furthermore, the hypervascular supply from the hepatic artery balances the reduced flow through the normally dominant portal supply, rendering the overall blood-pool density within the lesion unaltered in comparison to the surrounding normal liver tissue.[9] In our case also the RBC scan was normal. Since these lesions can contain areas of hemorrhage, fibrosis and/or necrosis, their heterogeneous morphology can confuse them with hepatocellular carcinoma. Hence even after extensive preoperative investigations the diagnosis may remain ominously inconclusive. Ultimately surgical resection is resorted to, especially in view of the malignant potential of some of these lesions.[10,11] It is often difficult to choose between long-term follow-up and surgical resection, because liver resection itself is associated with substantial mortality and morbidity.[12]
References
Discussion
Benign lesions of the liver like LCA are rare and can pose significant diagnostic challenge especially when they present uncommon variants.[5] Differentiation between FNH and LCA can be difficult. In the presence of a variant like TA, radiology findings may not be characteristic. Even needle biopsies can be unreliable and difficult to obtain, but they are an important part of investigations in such cases. TA resembles FNH due to its ductular reaction and nodular architecture; however lack of central scar, sinusoidal dilatation, naked arteries and frequent intralesional hemorrhages make it morphologically closer to LCA.[4] It appears that FNH and LCA comprise poles of a histological spectrum which ranges from fibrotic scar, arterioles and ductules in classic FNH to lack of fibrosis, naked arterioles and absence of ductular reaction in classic LCA, with TA lying somewhere between the two.[4]
LCA is overwhelmingly common in young women (92%) taking oral contraceptives and long duration of intake probably predisposes them to TA.2 This association however is not synonymous and LCA should not be construed as the precursor of TA, biological causality between the two being unclear. Other risk factors reported include obesity and inflammatory syndromes.[6] FNH is recognized with relative ease on imaging and once diagnosed requires no further treatment. Recently characterized radiological features of TA consists of a heterogeneous lesion with hyperintensity in T1-weighted MR images, strong hyperintensity in T2-weighted MR images, and persistent contrast enhancement in delayed-phase contrast enhanced CT or T1-weighted MRI with lack of central scar.[7] These findings were found to correlate well with histopathology features of TA.[5]
Much effort has been put to delineate the exact nature of this lesion. Molecular genetics of the neoplasm showed a much higher rate of monoclonality in TA compared to classic FNH. Although, the polyclonal nature of FNH argues against its neoplastic nature, as many as 50% tumors show X-chromosome inactivation suggestive of monoclonality, thus favoring a neoplastic nature.[8] Despite the demonstration of sinusoidal dilatation on histopathology and multiple tortuous, ectatic intralesional vessels on angiography, 99mTc labeled RBC studies often fails to detect any abnormality in TA. A probable explanation of this paradox is that although individual hepatic arteries in the lesion are enlarged and ectatic, they remain too small to be picked up by the 99mTc labeled RBC blood-pool technique. Furthermore, the hypervascular supply from the hepatic artery balances the reduced flow through the normally dominant portal supply, rendering the overall blood-pool density within the lesion unaltered in comparison to the surrounding normal liver tissue.[9] In our case also the RBC scan was normal. Since these lesions can contain areas of hemorrhage, fibrosis and/or necrosis, their heterogeneous morphology can confuse them with hepatocellular carcinoma. Hence even after extensive preoperative investigations the diagnosis may remain ominously inconclusive. Ultimately surgical resection is resorted to, especially in view of the malignant potential of some of these lesions.[10,11] It is often difficult to choose between long-term follow-up and surgical resection, because liver resection itself is associated with substantial mortality and morbidity.[12]
References
- Nguyen BN, Flejou JF, Terris B, Belghiti J, Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441–54.
- Paradis V, Benzekri A, Dargere D, Bieche I, Laurendeau I, Vilgrain V, et al. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126:1323–9.
- Bioulac-Sage P, Rebouissou S, Sa Cunha A, Jeannot E, Lepreux S, Blanc JF, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211–8.
- Yan BC, Hart JA. Recent developments in liver pathology. Arch Pathol Lab Med. 2009;133:1078–86.
- Attal P, Vilgrain V, Brancatelli G, Paradis V, Terris B, Belghiti J, et al. Telangiectatic focal nodular hyperplasia: US, CT, and MR imaging findings with histopathologic correlation in 13 cases. Radiology. 2003;228:465–72.
- Paradis V, Champault A, Ronot M, Deschamps L, Valla DC, Vidaud D, et al. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology. 2007;46:140–6.
- Takayassu TC, Marchiori E, Eiras A, Cabral RF, Cabral FC, Batista RR, et al. Telangiectatic adenoma - computed tomography and magnetic resonance findings: a case report and review of the literature. Cases J. 2009;2:24.
- Jain S, Singhal S, Lee P, Xu R. Molecular genetics of hepatocellular neoplasia. Am J Transl Res. 2010;2:105–18.
- Peterfy CG, Rosenthall L. Large telangiectatic focal nodular hyperplasia presenting with normal radionuclide studies: case report. J Nucl Med. 1990;31:2037–9.
- Larson KA, Weber SM, Fong Y, Blumgart LH. Malignant transformation of hepatic adenoma with recurrence after resection. HPB (Oxford). 2002;4:139–43.
- Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129:712–7.
- Colli A, Fraquelli M, Massironi S, Colucci A, Paggi S, Conte D. Elective surgery for benign liver tumours. Cochrane Database Syst Rev. 2007:CD005164.
|
|
|
 |
|
|