|
|
|
|
 |
 |
| |
 |
|
|
Editorial |
|
|
|
|
|
Keywords :
|
|
|
SK Mahiuddin Ahammed, Abhijit Chowdhury
Department of Hepatology,
School of Digestive and Liver
Diseases,
Institute of Post Graduate Medical
Education and Research,
244 AJC Bose Road,
Kolkata - 700020, India
Corresponding Author:
Dr. Abhijit Chowdhury
Email: achowdhury2002@yahoo.
co.in
DOI:
http://dx.doi.org/10.7869/tg.183
48uep6bbphidvals|642 48uep6bbph|2000F98CTab_Articles|Fulltext The word “cryptogenic” originates from the Greek word “kryptos”, meaning something hidden or secret. Cryptogenic and idiopathic are etiological expressions that denote relative paucity of knowledge regarding the cause of a disease at a given point of time. With scientific advancement and development of new diagnostic tools, cryptogenic disorders are better understood, diagnosed and managed. An aggressive investigative approach to determine the etiology of chronic hepatitis and cirrhosis is the current management strategy for these disorders. However, despite extensive investigations a significant fraction of patients still cannot be offered a definite diagnosis and are labeled as cryptogenic. It is noteworthy that both cryptogenic cirrhosis (CC) and non-alcoholicsteatohepatitis (NASH) are diagnosis of exclusion.Thus cirrhosis is labeled as cryptogenic only after exclusion of known causes like viral,alcoholic, drug-induced,metabolic and autoimmune causes for liver disease. Similarly, the term NASH is designated when clinical, anthropometric and pathological evidence exists and significant alcohol intake has been excluded. Temporally, these changes in CC and NASH are happening synchronously. The progress in understanding of pathophysiology, biology and natural history of NASH is occurring at the cost of CC. It still remains debatable if the two entities are essentially the same or it would be too reductionist to club them together.
Cryptogenic cirrhosis and NASH: a temporal trajectory
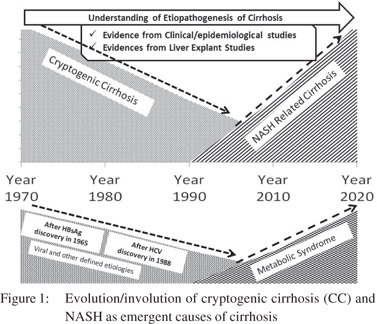
Crytpogenic cirrhosis was the predominant etiology of chronic hepatitis till 1965.[1] An elegant autopsy series from 1900 to 1969,and a study from India validate this observation.[2,3]The discovery of the hepatitis B virus(HBV) in 1965 and subsequent development of HBV serology were major breakthroughs in understanding CC.[4]Further refinement of serology techniques over the next two decades provided precision diagnosis and delineation of HBV natural history and also better diagnose autoimmune hepatitis, which contributed a significant burden of cryptogenic cirrhosis, besides HBV.The discovery of hepatitis C virus (HCV) in 1988 was the next watershed for management of chronic hepatitis and diagnosis of several CC cases.[5] Today cryptogenic cirrhosis is an infrequent diagnosis. In the United Network for Organ Sharing (UNOS) database(1988–1996),cryptogenic cirrhosis was diagnosed in 9.5% (1,726 of 18,172) patients.[6] In a Japanese epidemiological study, the prevalence of cryptogenic cirrhosis was found to be 9.9% among chronic liver disease patients, while other case series have reported cryptogenic cirrhosis in 5-30% patients, depending on the vigor applied to seek a specific etiology.[7-9]Fatty liver has long been associated with metabolic derangements, particularly diabetes. Evidence linking it with cirrhosis started emerging in the 1970s and by 1990 it definitive findings showed it as a precursor of cirrhosis.[10] Given that steatosis frequently disappears by the time cirrhosis develops in NASH and the lack of a precise biomarker, makes NASH-associated cirrhosis an as yet vague clinical entity frequently merged with CC. Lifestyle changes have increased the prevalence of obesity and metabolic syndrome.Consequently the frequency of NASHrelated cirrhosis is expected to increase over time. The impact is also evident in liver transplantation.Over a 6-year period,NASH-related cirrhosis contributed to 2.7% of all liver transplantations.[11] Subsequently between 1998-2003 NASHrelated cirrhosis contributed to 6.8% of all liver transplantations. A mathematical model comparing the impact of NAFLD and HCV on transplantation suggests that by 2020 NAFLD will surpass HCV as indication for liver transplantation.[11]
NASH-associated cirrhosis and cryptogenic cirrhosis: are they synonymous?
The natural history of NASH is characterized by indolent, progressive or steady inflammation and fibrosis of liver often leading to cirrhosis. Macro-vesicular steatosis is the histological hallmark of NASH and the primary difficulty in identifying NASH-associated cirrhosis is due the frequent disappearance of fat by the time cirrhosis gets established.[10] Thus the diagnosis of NASH-associated cirrhosis is primarily circumstantial and associative, rather than being based on any objective marker. Serial liver biopsies in NASH,along with evaluation of explants and follow-up of liver transplants in cryptogenic cirrhosis, uncovers the relationship between NASH-associated cirrhosis and CC.
In the most convincing evidence of NASH-associated cirrhosis, 42 biopsy proven steatohepatitis patients were followed-up for a median duration of 4.5 years. Interval biopsies revealed no change in 31%, whereas 14% showed progression including cirrhosis in one patient.[10]Most remarkably, fat and inflammation were absent in the presence of cirrhosis in these NASH patients. This “loss of footprint” was also documented in another study comparing sequential histological features of fatty liver-associated cirrhosis with hepatitis C cirrhosis.[12]
Presence or absence of steatosis failed to discriminate between NASH and HCV cirrhosis; although ballooned hepatocytes and Mallory-Denke bodies could stand as markers of NASH.[12]
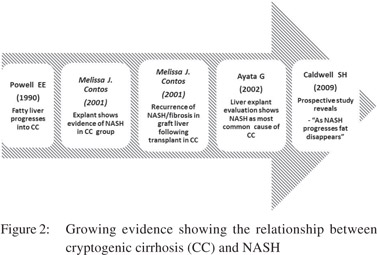
This disappearance of fat with progression of NASH is enigmatic. The possible factors include: loss of sinusoidal fenestrations that retard fat deposition in hepatocytes across the sinusoids; hepatocyte deprivation of the lipogenic hormones, particularly insulin, as a result of porto-systemic shunting;acinarre population by stem cell derived hepatocytes that are relatively disease resistant; and increased adiponectin levels exerting their potent anti-steatotic action.[13-17] In fact, studies have shown significant correlation between serum adiponectin levels and hepatic fat loss or the development of so called “burnt-out NASH”.[17] These studies were based on liver needle biopsies,but explants from cryptogenic cirrhotics undergoing liver transplantation, brings out the fatty liver component of cryptogenic cirrhosis with greater clarity. Steatosis (80%) and ballooning (70%) were indicative of NASH in 30 CC explants subjected to liver transplant.[18]Even more significant is the rate of reappearance of steatosis (100% in 5 years) in the allografts of cryptogenic cirrhosis, which is much higher than that occurring in allografts of alcoholics and those with PBC/PSC (25% at 5 years).[18] In addition, there was recurrence of full blown NASH in 10% explants, often with significant fibrosis.[18] A study of explants from cryptogenic cirrhosis has revealed that NASH was the commonest etiology (33%) followed by cryptic autoimmunity (22%) and alcohol abuse (22%).[19] An explant based study from India included 84 cases of cryptogenic cirrhosis and found NAFLD as the cause of cirrhosis in 57 patients and non-cirrhotic portal fibrosis in nine patients.[20] Another study from India assessed pathomorphological feature of explants and showed 63% of cryptogenic cirrhosis is NASH related.[21]
The demographic factors and co-morbidities of NASH and CC also overlap. Cryptogenic cirrhosis is predominantly seen in women and it usually present between 55-60 years of age. Frequency of obesity (45-48%)and diabetes (40-50%) is also much higher in cryptogenic cirrhosis compared to controls.[22] The frequency of obesity,diabetes mellitus and dyslipidemiafeatures of metabolic syndrome - are much higher in cryptogenic cirrhosis than virus-associated cirrhosis.[23] Both obesity and type 2 diabetes mellitus are inheritable disorders. Hence NASH and cryptogenic cirrhosis may also have similar genetic basis, as has been demonstrated by Struben et al.[24] In fact this study has shown NASH and cryptogenic cirrhosis to be part of the same disease spectrum. The age difference between patients with nonalcoholic steatohepatitis without cirrhosis, steatohepatitis and cirrhosis, and those with cryptogenic cirrhosis suggests a gradual progression of this disorder over many years.[24]In this study nonalcoholic steatohepatitis and nonalcoholic steatohepatitis with cirrhosis coexisted within four kindred, one of who also had a family member afflicted with cryptogenic cirrhosis. Nonalcoholic steatohepatitis and cryptogenic cirrhosis coexisted within three additional kindred.[24] The coexistence of nonalcoholic steatohepatitis with and without cirrhosis with cryptogenic cirrhosis within these families suggests common underlying pathogenesis and a possible genetic link. A number of studies have explored the role of different genes in the pathogenesis of steatosis. Of these PNPLA3 (patatinlike phospholipase 3) and apolipoprotein C3 deserve special mention.[25,26] A synergistic association between angiotensinogen and transforming growth factor beta 1 has been described in patients with severe NASH.[27] The identification of the same gene polymorphism in cryptogenic cirrhosis might further indicate it as a sequel of NASH.
Cryptogenic cirrhosis and autoimmune hepatitis
Several studies have suggested that burnt-out autoimmunue hepatitis (AIH) can also lead to cryptogenic cirrhosis.[28] Autoimmune hepatitis has a variable clinical phenotype and may lack conventional autoantibodies or the autoantibodies may disappear with time.[29,30] The frequency of presumed autoantibody-negative autoimmune hepatitis in patients with acute presentations is 7%, and its frequency in patients with chronic presentations is 1-34%.[31] A Turkish group treated two groups of patients with cryptogenic chronic hepatitis and AIH with similar clinical and biochemical features, with prednisolone and azathioprine for two years.[32] They found similar remission rates and human leukocyte antigen (HLA) phenotypes in both the groups. Many other studies which have shown that cryptogenic liver disease can resemble classical autoimmune hepatitis by gender, age, laboratory indices, histological findings, HLA, and response to corticosteroid therapy.[33,34] In the absence of antinuclear antibody(ANA) and smooth muscle antibody (ASMA),new serological markers of AIH including soluble liver antigen antibodies (anti-SLA), perinuclear antineutrophil cytoplasmic antibodies (pANCA), and antibodies to liver specific membrane lipoprotein or asialoglycoprotein receptor (ASGPR) can further diagnose 18–25% cases of CC.[34]
Cryptogenic cirrhosis and occult HBV infection
Occult HBV infection is defined as the presence of HBV viral genome in liver tissue with or without detectable serum HBV DNA in absence of HBsAg. A key question is whether the presence of small amounts of HBV can lead to progressive liver disease. Available data suggests that the likelihood is extremely low. Among patients with persistent low levels of HBV DNA after apparent recovery from acute hepatitis B, there is no evidence that they have increased risks of cirrhosis or hepatocellular carcinoma (HCC). Cirrhosis and HCC have been reported in patients with seropositive occult HBV infection, but it is not clear if the cirrhosis or HCC is a result of persistent low levels of HBV or other causes of liver disease. In patients with other etiologies of chronic liver disease such as alcoholism and hepatitis C virus infection, HBV may be a bystander or a cofactor in the pathogenesis of liver disease. One study carried out liver biopsy in patients who had elevated ALT of unknown etiology.[35] Liver biopsies from these patients showed nonspecific changes in 32.7% of cases, NASH in 15.8%, and chronic hepatitis or cirrhosis in 51.5%.[35] HBV-DNA and/or HCV-RNA was detected more frequently in cryptogenic liver disease than in healthy blood donors (26.7% vs. 3.4%; p<0.001).[35] The proportion of cases with detectable HBV-DNA or HCV-RNA was 14.3% in patients with non-specific changes or NASH, 30.7% in patients with chronic hepatitis, and 61.5% in patients with cirrhosis.[35] Cirrhosis was found more frequently in patients with positive HBV-DNA and/or HCV-RNA in serum than in those who tested negative (p=0.005).35 In another study among 1,355 chronic carriers from 1985 to1997,spontaneous HBsAg clearance was observed in 55 patients. During a mean followup of 23 months, 18(32.7%; all were male subjects) developed serious complications,including 11 cases of hepatocellular carcinoma (HCC), six cirrhosis, and one sub-fulminant liver failure.[36] The overall cumulative probability of complications was 29.8% at 4 years.[36] In another study out of 189 patients who were non-cirrhotic at the time of HBsAg clearance,3(1.6%) developed cirrhosis,2(1.1%)developed hepatocellular carcinoma (HCC), and one died of HCC.[37] These complications developed in patients with concomitant hepatitis C virus or hepatitis delta virus infection (p<0.001)[37]. A study from India tested sera of 111 patients with cirrhosis. Of 18 patients labeled as ‘cryptogenic’ by serological testing, HBV-DNA was detected in the serum of seven patients. Of 14 patients in whom paired liver tissue and serum specimens were tested, four additional patients with HBV infection were detected after liver biopsy.[38]The prevalence of occult HBV infection in general population is 15-18%.[39] As fatty liver and occult HBV infection both are common in the general population, a patient may have both fatty liver and occult HBV infection. In such patients it is difficult to establish the cause of cirrhosis. So it is reasonable to screen patients with cryptogenic liver disease for occult HBV infection assuming that a small proportion of patients with cryptogenic liver disease may have occult HBV infection, especially in areas of high HBV prevalence.
Alcohol and cryptogenic cirrhosis
Diagnosis of NAFLD relies heavily on exclusion of alcohol related liver injury and histopathology consistent with NAFLD. Risk of alcoholic liver disease is not increased until a patient starts drinking more than 30 grams of alcohol per day. Most NAFLD studies have used this threshold to differentiate between alcohol and non-alcoholic fatty liver disease. Obtaining a history of alcohol intake is fraught with many limitations. Firstly, patients may be reluctant to give history of alcohol intake. Secondly, inadequate history of alcohol intake in a busy outpatient department might yield false negative information. Third element in this controversy is the reliance on recall resulting in underestimation of alcohol intake. This may label a true alcoholic liver disease as NAFLD or cryptogenic. So routine history taken by the physician may be less accurate at identifying such patients. One study measured current and total life time alcohol intake in biopsy proven NAFLD patients using cognitive life time drinking history (CLDH), a computerized questionnaire. In this study CLDH revealed significant past alcohol intake in 13% patients which was missed by standard questionnaires.[40] So occult alcohol intake may be an important cause of cryptogenic cirrhosis. A study on explants from cryptogenic cirrhosis has revealed alcohol abuse (22%) as an important cause of cryptogenic cirrhosis.[19]
Summary
Cryptogenic cirrhosis is a clinical entity which has the potential for better diagnosis of actual underlying maladies with future advancements in medicine. Current understanding of CC is rapidly undergoing transformation (Figures 1 & 2). In the light of available evidence of frequent disappearance of fat in NASHassociated cirrhosis, CC explants showing steatosis, allografts demonstrating the full blown recurrence of NASH in CC, and the association of CC with diabetes and obesity, suggests CC to be more proximately linked with NASH than believed until now (Figure 2). However, there are subset of patients within CC that appear to emerge from other etiologies. With increasing clarity of NASH pathogenesis, a useful biomarker might soon be available.Advancements in diagnostics and our understanding of NASH are likely to elucidate NASHassociated cirrhosis as the predominant cause of cryptogenic cirrhosis.
 References
References
- Popper H,Schaffner F. Progress in liver disease. Volume 6. Pennsylvania State University: Grune& Stratton; 1979.
- MacSween RN, Scott AR. Hepatic cirrhosis: a clinico-pathological review of 520 cases. J Clin Pathol. 1973;26:936–42.
- Kasliwal RM, Sharma BM, Chaturvedi GC. Aetiological factors of cirrhosis of the liver in adults in India. A study of 290 cases. J Indian Med Assoc. 1965;44:407–13.
- Blumberg BS, Alter HJ, Visnich S. A “New” antigen in leukemia sera. JAMA. 1965;191:541–6.
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62.
- Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–9.
- Sakugawa H, Nakasone H, Nakayoshi T, Kawakami Y, Yamashiro T, Maeshiro T, et al. Clinical characteristics of patients with cryptogenic liver cirrhosis in Okinawa, Japan. Hepatogastroenterology. 2003;50:2005–8.
- Kodali VP, Gordon SC, Silverman AL, McCray DG. Cryptogenic liver disease in the United States: further evidence for non-A, non-B, and non-C hepatitis. Am J Gastroenterol. 1994;89:1836–9.
- Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed). 1981;282:263–6.
- Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80.
- Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–58.
- Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346–52.
- Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–42.
- Matsui O, Kadoya M, Takahashi S, Yoshikawa J, Gabata T, Takashima T, et al. Focal sparing of segment IV in fatty livers shown by sonography and CT: correlation with aberrant gastric venous drainage. AJR Am J Roentgenol. 1995;164:1137–40.
- Nosadini R, Avogaro A, Mollo F, Marescotti C, Tiengo A, Duner E, et al. Carbohydrate and lipid metabolism in cirrhosis. Evidence that hepatic uptake of gluconeogenic precursors and of free fatty acids depends on effective hepatic flow. J Clin Endocrinol Metab. 1984;58:1125–32.
- Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–11.
- van der Poorten D, Samer CF, Ramezani-Moghadam M, Coulter S, Kacevska M, Schrijnders D, et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57:2180–8.
- Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–73.
- Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, et al. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol. 2002;33:1098–104.
- Nayak NC, Jain D, Vasdev N, Gulwani H, Saigal S, Soin A. Etiologic types of end-stage chronic liver disease in adults: analysis of prevalence and their temporal changes from a study on native liver explants. Eur J Gastroenterol Hepatol.2012;24:1199–208.
- Nayak NC, Vasdev N, Saigal S, Soin AS. End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol. 2010;41:425–30.
- Maheshwari A, Thuluvath PJ. Cryptogenic cirrhosis and NAFLD: are they related? Am J Gastroenterol. 2006;101:664–8.
- Duseja A, Sharma B, Kumar A, Kapil S, Das A, Dhiman RK, et al. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatology. 2010;52:2248–9.
- Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13.
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5.
- Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–9.
- Dixon JB, Bhathal PS, Jonsson JR, Dixon AF, Powell EE, O’Brien PE. Pro-fibrotic polymorphisms predictive of advanced liver fibrosis in the severely obese. J Hepatol. 2003;39:967–71.
- Kaymakoglu S, Cakaloglu Y, Demir K, Turkoglu S, Badur S, Gurel S, et al. Is severe cryptogenic chronic hepatitis similar to autoimmune hepatitis? J Hepatol. 1998;28:78–83.
- Czaja AJ, Carpenter HA, Santrach PJ, Moore SB, Homburger HA. The nature and prognosis of severe cryptogenic chronic active hepatitis. Gastroenterology. 1993;104:1755–61.
- Czaja AJ. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol. 1999;30:394–401.
- Czaja AJ. Autoantibody-negative autoimmune hepatitis. Dig Dis Sci. 2012;57:610–24.
- Czaja AJ, Hay JE, Rakela J. Clinical features and prognostic implications of severe corticosteroid-treated cryptogenic chronic active hepatitis. Mayo Clin Proc. 1990;65:23–30.
- Gassert DJ, Garcia H, Tanaka K, Reinus JF. Corticosteroidresponsive cryptogenic chronic hepatitis: evidence for seronegative autoimmune hepatitis. Dig Dis Sci. 2007;52:2433–7.
- Czaja AJ. Cryptogenic chronic hepatitis and its changing guise in adults. Dig Dis Sci. 2011;56:3421–38.
- Berasain C, Betes M, Panizo A, Ruiz J, Herrero JI, Civeira MP, et al. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut. 2000;47:429–35.
- Huo TI, Wu JC, Lee PC, Chau GY, Lui WY, Tsay SH, et al. Seroclearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology. 1998;28:231–6.
- Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–9.
- Agarwal N, Naik S, Aggarwal R, Singh H, Somani SK, Kini D, et al. Occult hepatitis B virus infection as a cause of cirrhosis of liver in a region with intermediate endemicity. Indian J Gastroenterol. 2003;22:127–31.
- Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini- Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194–203.
- Hayashi PH, Harrison SA, Torgerson S, Perez TA, Nochajski T, Russell M. Cognitive lifetime drinking history in nonalcoholic fatty liver disease: some cases may be alcohol related. Am J Gastroenterol. 2004;99:76–81.
|
|
|
 |
|
|