|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
chronic pancreatitis, bone mineral density, bone mineral metabolism |
|
|
Anusree Prabhakaran,1 Deepak K Bhasin,2 Surinder S Rana,2 Sanjay K Bhadada,3 Anil Bhansali,3 Chalapathi Rao,2 Rajesh Gupta,4 Niranjan Khandelwal5
Departments of Internal Medicine,1
Gastroenterology,2 Endocrinology,3
Surgery4 and Radiodiagnosis5
Post Graduate Institute of Medical
Education and Research (PGIMER),
Sector 12, Chandigarh – 160012,
India
Corresponding Author:
Dr. Surinder S Rana
Email: drsurinderrana@yahoo.co.in
DOI:
http://dx.doi.org/10.7869/tg.189
Abstract
Background: There is limited information on the bone mineral metabolism in patients with chronic pancreatitis (CP).
Methods: 103 patients with CP (all males; mean age 38.6±20.64 yrs) and 40 age matched control males (mean age: 36.7±20.70 yrs) were prospectively studied. Serum levels of 25 (OH) Vitamin D3, alkaline phosphatase (ALP), and parathyroid hormone (PTH) were measured. Bone mineral density (BMD) was measured using a dual-energy X-ray absorptiometry (DEXA) scanner.
Results: Seventy two (70%) patients had alcohol related chronic pancreatitis (ACP), 30 (29.1%) patients had idiopathic chronic pancreatitis (ICP) and one patient had post-traumatic chronic pancreatitis. Fifty nine (59.8%) patients had chronic calcific pancreatitis (CCP) and 39 (37.8%) patients were diabetic. Steatorrhea was noted in 21 (20.4%) patients. On comparison with controls, patients with chronic pancreatitis had significantly lower 25 (OH) Vitamin D3 levels (p=0.01). On evaluation of bone mineral density (BMD) at lumbar spine, 46% patients were osteopenic and 12% patients were osteoporotic. On evaluation of BMD of femur, 30.1% patients were osteoporotic and 39.8% patients were osteopenic. No significant difference was found in the frequency of metabolic osteopathy between alcoholic and idiopathic groups (p=0.108), calcific and non-calcific groups (p=0.410), diabetic and non-diabetic groups
(p=0.126), smokers and non-smokers (p=0.198), and patients with and without history of steatorrhea (p=0.265) and indifferent severity groups of pancreatitis (p=0.910).
Conclusions:Majority of patients with both ACP and ICP had low BMD and similar frequency of bone changes between various groups suggests that systemic inflammation may play an important role in its pathogenesis. Further detailed metabolic studies are necessary to define the pathogenic mechanism of metabolic osteopathy associated with chronic pancreatitis.
|
48uep6bbphidvals|648 48uep6bbph|2000F98CTab_Articles|Fulltext Chronic pancreatitis is characterized by irreversible damage to the pancreas that eventually leads to pain and/or exocrine and/or endocrine insufficiency.[1]Exocrine insufficiency leads to malabsorption and nutritional deficiency especially of fat soluble vitamins. Vitamin D deficiency due to impaired absorption can result in abnormal bone mineral metabolism and osteoporosis. It has been demonstrated that patients with pancreatic insufficiency have low serum concentrations of vitamin D due to impaired absorption and its deficiency is one of the important factors in development of bone changes in patients with chronic pancreatitis.[2-4]
However, impaired fat absorption and vitamin D malabsorption are not the only pathogenic mechanisms contributing to impaired bone mineral metabolism in chronic pancreatitis. Other pathogenic mechanisms like alcoholism,reduced dietary intake of calcium and vitamin D and underlying chronic inflammatory state have also been proposed as likely causes for abnormal bone metabolism in patients with chronic pancreatitis. The elevated levels of systemic inflammation in chronic pancreatitis disrupt bone mineral metabolism because bone-mass turn over depends on the dynamic equilibrium between pro-and anti-inflammatory pathways.[4-6]
Alterations in calcium metabolism have been reported earlier as well but in small cohorts of male chronic pancreatitis patients.[7-9] Only few studies have reported alterations in bone mineral density and as well as increased risk of fractures in these patients.[4,7-9] However, very few studies have attempted to correlate the severity of chronic pancreatitis with decreased serum vitamin D levels and bone mineral density. In the same context, bone mineral metabolism in patients with idiopathic chronic pancreatitis (ICP) has been inadequately studied.10A study from India reported a high frequency of low bone mineral density (BMD) in patients with tropical chronic pancreatitis (TCP).[10] These TCP patients had significantly lower body mass index (BMI) and serum albumin levels than the controls, suggesting that malnutrition is an important factor contributing to abnormal bone mineral metabolism in these patients.10 We have recently reported that the clinical profile of ICP in north India differs from the classical TCP described in literature.Our patients showed normal BMI with higher incidence of pain and lower frequencies of diabetes, calcification and intraductal calculi.[11,12]These differences in ICP profile are probably due to the change in environment,dietary habits and nutritional status which have accompanied the economic development over last two decades.Since vitamin D deficiency is not the only factor disrupting bone mineral metabolism in ICP patients, it is pertinent to examine the effect improved nutritional status has on this disease.We prospectively studied BMD, vitamin D status and bone mineralization parameters in 103 patients with chronic pancreatitis and compared them between patients with alcohol related chronic pancreatitis (ACP) and idiopathic chronic pancreatitis (ICP).
Methods
One hundred and three male patients with chronic pancreatitis presenting to the Gastroenterology, Surgical Gastroenterology and Endocrinology services of our Institute, between January 2010 and May 2011 were prospectively enrolled for this study. The study was approved by our Institute Ethics Committee.An informed consent was obtained from all patients and controls at enrolment. Chronic pancreatitis was diagnosed clinically and by biochemical and radiological investigations.1All patients with chronic abdominal pain were thoroughly evaluated and chronic pancreatitis was confirmed by pancreatic calcifications on abdominal X-ray, ultrasonography and/or abdominal computed tomography;and by characteristic ductal changes on magnetic resonance cholangiopancreatography (MRCP) and/or endoscopic retrograde cholangiopancreatography (ERCP). A detailed history specifically assessing the family history, alcohol consumption and presence and severity of abdominal pain was recorded. The absence of alcohol consumption was confirmed by repeated interviews of the patient as well as of the family members. Patients with gastrointestinal diseases causing malabsorption, chronic inflammatory diseases, chronic renal failure and patients on drugs including long-term steroid therapy, phenytoin, ketoconazole, antitubercular therapy, thyroid hormone replacement, regular calcium and vitamin D supplementation were excluded.
Enrolled patients were classified as calcific or non-calcific pancreatitis based on radiological evidence.Chronic pancreatitis patients consuming more than 50g alcohol per day for at least 5 years were diagnosed as alcohol induced chronic pancreatitis.13ICP was diagnosed if all other causes of chronic pancreatitis including hypertriglyceridemia, primary hyperparathyroidism, abdominal trauma, post-surgical pancreatic duct stenosis, hereditary pancreatitis (determined by family history), and excessive alcohol consumption were ruled out.[13]All the patients were subjected to a thorough clinical examination, routine hematological and biochemical investigations, abdominal X-ray, ultrasonography, and computed tomography (CT). Additional investigations like magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS), glucose tolerance test (GTT) and fecal fat studies were performed when indicated. ERCP was performed in patients who were treated with pancreatic endotherapy.The severity of chronic pancreatitis was assessed by ductal criteria (Cambridge Classification)[14]and the patients were divided into mild, moderate and severe pancreatitis.
Serum 25 hydroxyvitamin D3 was estimated by radioimmunoassay (BioSource Europe SA; inter-assay CV 5.2%, intra-assay CV 3.3-4.7%, assay detection limit 2.1-154ng/ml) and serum intact parathormone (iPTH) was estimated by immuno-chemiluminescenceassay (Elecsys 2010, Roche, Germany, CV=4.3-7.1%). Serum vitamin D levels were considered to be sufficient above 30ng/ml, insufficient between 10-30ng/ ml and deficient if less than 10ng/ml.15 The samples for biochemistry were analyzed within 12 hours of presentation to the hospital whereas sera for 25(OH)D3, and iPTH were stored at -70oC until measurement.Bone mineral density was measured by dual-energy x-ray absorptiometry (DEXA) scan of the lumbar spine and femoral neck (Norland XR-46, Cooper Surgical Inc, Trumbull CT, USA).The T-score was used to diagnose whether a particular patient had osteopenia or osteoporosis. A T-score less than -2.5 indicated osteoporosis and between -1.0 to -2.5 demarcated osteopenia.
Controls
Forty age-matched healthy adult males were enrolled as healthy controls after informed consent. None of these subjects had hepatic or renal dysfunction, malabsorption, or were receiving drugs that affected vitamin D metabolism. All of them underwent a thorough clinical examination as well as routine hematological and biochemical investigations. Their blood parameters were within normal limits.Their sera was collected for estimation of 25(OH)D3 and iPTH, and was stored at -70ÚC until measurement.
Statistical analysis All quantitative variables were expressed as mean± standard deviation or median with range, as appropriate. Categorical variables were comparison using Chi-square test. Continuous variables across different groups were compared using independent t-test and one way analysis of variance for more than two groups. A p value <0.05 was taken as significant.
Results
One hundred and three male patients (mean age: 38.6±20.64 years; range 18-60 years) with chronic pancreatitis were included in the study and 40 age-matched healthy males (mean age: 36.7±20.70 years; range 18-67 years) comprised the control group.Alcohol consumption was the most common cause of chronic pancreatitis (n=72; 69.9%); followed by ICP (n=30; 29.1%) and one patient had post-traumatic chronic pancreatitis. Fifty nine (57.2%) patients had calcific pancreatitis and 44 (42.8%) patients had non-calcific pancreatitis.Pain was the most common presenting symptom (n=102; 99%) with a mean duration of 49.9±138.82 months. The mean BMI of the chronic pancreatitis patients was 19.7kg/m2 (range: 11- 28.97kg/m2). The mean duration of sunlight exposure was 2.6±5.80 hours per day. Out of these 103 patients, 39 (37.8%) patients were diabetic and 21 (20.4%) gave history of steatorrhea.As per the Cambridge Classification majority of the patients had severe pancreatitis. Complete break-up of cases included mild, moderate and marked pancreatitis changes in 13 (13.1%), five (5.05%) and 81 (81.8%) patients, respectively.
Vitamin D and iPTH levels
Serum 25-(OH) vitamin D3 levels were tested in 94 patients.The stored sera of remaining nine patients were found unfit for analysis. Three patients had supra-normal values and they were not included in the analysis. The remaining 91 patients had a mean serum 25(OH) vitamin D3 level of 27.57±52ng/ml with minimum being 4ng/ml and maximum 100ng/ml. 25(OH) vitamin D3 was deficient in 20 (19.4%) patients, insufficient in 42 (40.8%) patients and normal in 29 (28.2%) patients.Serum iPTH levels were measured in 94 patients and the mean serum iPTH was 27.6±39.8 pg/ml with minimum and maximum levels at 3.6 and 144 pg/ml, respectively. Twenty (21.3%) patients had low, 69 (73.4%) patients had normal and five (5.1%) patients had high iPTH levels.The mean serum 25(OH) vitamin D3 level in the patient group was significantly lower than that in the control group (27.57±52ng/ml vs. 38.59±26ng/ml; p=0.013). Although the mean iPTH level in the patient group was higher than that in the control group, but the difference was not statistically significant (27.6±39.8 pg/ml vs. 27.5±34.22 pg/ml; p=0.424).
Bone mineral density
The BMD was estimated in 83 patients while others refused to undergo a DEXA scan. Lumbar spine T-scores revealed 38 (45.8%) patients to be osteopenic and 10 (12%) patients were osteoporotic. Remaining 35 (42.2%) patients had normal spinal BMD. Femur T-scores showed 33 (39.8%) patients to be osteopenic and 25 (30.1%) were osteoporotic. Remaining 35 (42.2%) patients had normal femoral BMD. The mean lumbar spine T-score was -1.14±2.68 and the mean femoral T-score was-1.69±2.66.
Bone metabolism parameters across various grades of pancreatitis
The mean serum 25(OH) vitamin D3 levels in patients with mild, moderate and marked changes of chronic pancreatitis were 21.2±51.26ng/ml, 32.9±54.06ng/ml and 22.5±36.16ng/ml, respectively and this difference was not statistically significant (p=0.49). The mean serum 25(OH) vitamin D3 level in the calcific group was 26.4±38.70 ng/ml and that in the non-calcific group was 17.9±38.0 ng/ml with the difference being statistically insignificant (p=0.47). In patients with chronic calcific pancreatitis, 31.3% patients had normal femoral BMD whereas 39.6% patients had osteopenia and 29.2% had osteoporosis.On the other hand patients with non-calcific chronic pancreatitis, 30.3% had normal femoral BMD, 39.4% had osteopenia and 30.3% had osteoporosis (p=0.993). Similarly in patients with chronic calcific pancreatitis, 39.6% had normal lumbar spine BMD, 45.8% had osteopenia and 14.6% had osteoporosis. In non-calcific pancreatitis, 42.4% had normal lumbar spineBMD, 48.5% had osteopenia and 9.1% had osteoporosis (p=0.761).
Bone metabolism parameters in ACP vs. ICP
The mean 25(OH)vitamin D3 level in the ACP group was 23.2±38.72ng/ml and that in the ICP group was 24.6±44.18ng/ ml (p=0.237). Similarly the mean iPTH level in ACP patients was 29.1±43.50pg/ml and in ICP patients was 26.3±26.86pg/ml (p= 0.241). In patients with ACP, the femoral BMD was normal in 14 (25%) patients whereas 23 (41%) patients had osteopenia and 19 (33.9%) had osteoporosis. On the other hand in patients with ICP, the femoral BMD was normal in 11 (40.7%) patients, 11 (40.7%) had osteopenia and five (18.5%) had osteoporosis (p=0.224).In ACP patients, the lumbar spine BMD was normal in 20 (36.4%) patients, 27 (49.1%) had osteopenia and eight (14.5%) had osteoporosis. In contrast ICP patients had normal lumbar spine BMD in 14 (51.9%) patients,while 11 (41.2%) had osteopenia and two (7.4%) showed osteoporosis (p=0.224).
Vitamin D status and bone mineral density
The mean femoral T-score in patients with vitamin D deficiency was -1.82±2.2 and in patients with normal vitamin D levels was -1.71±3.2 (p=0.23). The mean spinal T-score in vitamin D deficient pancreatitis patients was -0.94±2.8 and that invitamin D sufficient patients was -1.24±2.6 (p=0.86). Furthermore, among patients with vitamin D deficiency, three (16.7%) had normal femoral BMD, 10 (55.6%) had osteopenia and five (27.8%) had osteoporosis. In the vitamin D insufficient group, 15 (44.1%) had normal femoral BMD, 11 (32.4%) had osteopenia and eight (23.5%) had osteoporosis. In the vitamin D sufficient group, five (25%) had normal BMD, 10 (50%) had osteopenia and five (25%) had osteoporosis (p=0.28).
Metabolic osteopathy in chronic pancreatitis
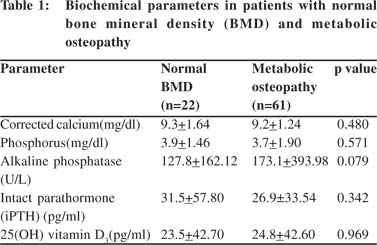
We analyzed patients with osteoporosis and osteopenia together as patients suffering from metabolic osteopathy. The calcium, phosphorous, alkaline phosphatase, iPTH and vitamin D levels were comparable between patients with metabolic osteopathy and those with normal BMD (Table 1). Further, there was no difference in the frequency of metabolic osteopathy between alcoholic and idiopathic groups (p=0.108), calcific and non-calcific groups (p=0.410), diabetics and nondiabetics (p=0.126), smokers and non-smokers (p=0.198), patients with and without history of steatorrhea (p=0.265) and amongdifferent pancreatitis severity groups (p=0.910).
Discussion
Despite being a common disease little attention has been paid to osteopenia in chronic pancreatitis.The bone mineral metabolism in chronic pancreatitis has been assessed by few studies.[7-9]Moreover, majority of these studies have enrolled patients with ACP and very few studies have examined BMD in ICP patients.[4,10]All these studies have shown that BMD is reduced significantly in patients with chronic pancreatitis and these patients are at increased risk of bone fractures.Our study corroborates that nearly two-thirds of our chronic pancreatitis patients had metabolic bone disease and these patients are at increased risk of bone fractures.
Dujsikova et al studied 73 patients (17 females and 56 males) in different stages of chronic pancreatitis and found that osteopathy was seen in 39% chronic pancreatitis patients (osteopenia in 26%, osteoporosis in 5% and osteomalacia in 8%).2Majority of these patients had ICP (89%) and the severity of pancreatitis was graded using endoscopic ultrasound criteria.Their study group consisted of 41 patients (56.2%) with mild, 12 patients (16.4%) with moderate and 20 patients (27.4%) with severe CP. Other studies have reported much higher frequency of bone changes in chronic pancreatitis (60- 90%).7,9However, these cohorts largely comprised of patients with advanced chronic pancreatitis in contrast to the study by Dujsikova et al where 56% patients had mild pancreatitis. In our study as well, majority of the patients with marked changes of chronic pancreatitis, showed metabolic osteopathy in nearly two-thirds of the cohort.
Mann et al examined 42 chronic pancreatitis male patients, with majority suffering from ACP.They found that BMD in these patients was significantly lower than that in controls.They also observed a significant correlation between increasing severity of chronic pancreatitis (determined byERCP and fecal elastase 1 levels)and decreased vitamin D3levels and decreased BMD.[16] In a cross-sectional study, Joshi et al studied 72 patients with tropical chronic pancreatitis (TCP) and found that their lumbar spine and total hip BMD were significantly lower than that in the controls.[10] They also found that vitamin D deficiency was equally prevalent among patients (86%) and controls (85%).However, we did not find any statistically significant correlation between chronic pancreatitis severity based on the Cambridge Criteria and the average spinal and femoral T-scores in our patients. There was also no significant difference in the number of patients with osteoporosis and osteopenia across different Cambridge groups. These observations could have been confounded by thesmall number of patients in the mild and moderate Cambridge grade. Bone health is affected by many other factors which could also have confoundedour results, like age, sunlight exposure, the percentage of surface area exposed to sunlight, dressing habits, dietary habits and lifestyle.
Mann et al also demonstrated that chronic pancreatitis patients had significantly lower vitamin D3levels when compared with controls, and with increasing severity of chronic pancreatitis, the vitamin D3 levels kept decreasing.[16]Other studies have alsoreported similarly lower vitamin D3 levels in chronic pancreatitis patients.[4-9] We also found significantly lower mean serum 25 (OH) vitamin D3 level in our CP patients as compared to the controls. However, these levels did not show any statistically significant variation across patients with mild, moderate and marked changes of chronic pancreatitis.This could also be confounded by the small mild and moderate Cambridge groups.Only 20% patients had steatorrhea.
A recent meta-analysis of 10 studies including 513 CP patients by Duggan et al found a pooled prevalence of osteoporosis at 23.4%, for osteopenia at 39.8% and for either condition at 65%.[17] They concluded that nearly 1 in 4 patients with chronic pancreatitis have osteoporosis, and almost twothirds have either osteoporosis or osteopenia.
An interesting finding in our study was the statistically similar frequency of metabolic osteopathy seen between alcoholic and idiopathic groups, calcific and non-calcific groups, diabetics and non-diabetics, smokers and non-smokers, patients with and without history of steatorrhea, patients with and without vitamin D deficiency and in different pancreatitis severity groups. This suggests that underlying chronic inflammation plays a predominant role in abnormal bone mineral metabolism in chronic pancreatitis. Experimental models of inflammatory bowel disease have demonstrated immunemediated bone changes mediated by various inflammatory cytokines such as tumor necrosis factor-á, interleukins (IL-1â, IL-6) and interferon-ã.[18-20] Although the exact pathogenic mechanisms responsible for bone changes in chronic pancreatitis have still not been resolved, elevations in proinflammatory cytokines like IL-1, IL-6 and tumor necrosis factor-á have been described in CP patients.[20-22] Bone turnover is strongly regulated by the interactions between the receptor activator of nuclear factor-kB (RANK), the RANK ligand and osteoprotegerin.This system represents a crucial link between bone turnover and circulating cytokines. An increased proinflammatory state, as seen in chronic pancreatitis, can shift bone metabolism toward resorption through the RANK ligand system.[4,23]
A large prospectively evaluated cohort of chronic pancreatitis patients is our study’s strength,althoughthe lack of BMD scores in controls and fewer patients with mild and moderate chronic pancreatitis are important limitations of our study.
In conclusion, majority of patients with both ACP and ICP had low BMD and the frequency of bone changes were similar between calcific and non-calcific groups, diabetics and nondiabetics, patients with and without history of steatorrhea, patients with and without vitamin D deficiency and across different pancreatitis severity groups.Our findings suggest that systemic inflammation may play an important role in inducing these changes. Further comprehensive metabolic studies are necessary to elucidate the pathogenic mechanism of metabolic osteopathy associated with chronic pancreatitis.
References
- Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis,classification, and new genetic developments. Gastroenterology.2001;120:682–707.
- Dujsikova H, Dite P, Tomandl J, Sevcikova A, Precechtelova M. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology. 2008;8:583–6.
- Vogelsang H, Schofl R, Tillinger W, Ferenci P, Gangl A. 25- hydroxyvitamin D absorption in patients with Crohn’s disease and with pancreatic insufficiency. Wien Klin Wochenschr. 1997;109:678–82.
- Bang UC, Benfield T, Bendtsen F, Hyldstrup L, Beck Jensen JE. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol. 2014;12:320–6.
- Gansauge F, Gansauge S, Eh M, Schlosser W, Ramadani M, Kern P, et al. Distributional and functional alterations of immunocompetent peripheral blood lymphocytes in patients with chronic pancreatitis. Ann Surg. 2001;233:365–70.
- Pezzilli R. Chronic pancreatitis: maldigestion, intestinal ecology and intestinal inflammation. World J Gastroenterol.2009;15:1673–6.
- Moran CE, Sosa EG, Martinez SM, Geldern P, Messina D, Russo A, et al. Bone mineral density in patients with pancreatic insufficiency and steatorrhea. Am J Gastroenterol. 1997;92:867–71.
- Mann ST, Stracke H, Lange U, Klor HU, Teichmann J. Vitamin D3 in patients with various grades of chronic pancreatitis, according to morphological and functional criteria of the pancreas. Dig Dis Sci. 2003;48:533–8.
- Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21–7.
- Joshi A, Reddy SV, Bhatia V, Choudhuri G, Singh RK, Singh N, et al. High prevalence of low bone mineral density in patients with tropical calcific pancreatitis. Pancreas. 2011;40:762–7.
- Bhasin DK, Singh G, Rana SS, Chowdry SM, Shafiq N, Malhotra S, et al. Clinical profile of idiopathic chronic pancreatitis in North India. Clin Gastroenterol Hepatol. 2009;7:594–9.
- Chari ST, Mohan V, Jayanthi V, Snehalatha C, Malathi S, Viswanathan M, et al. Comparative study of the clinical profiles of alcoholic chronic pancreatitis and tropical chronic pancreatitis in Tamil Nadu, south India. Pancreas. 1992;7:52–8.
- Gabbrielli A, Mutignani M, Pandolfi M, Perri V, Costamagna G. Endotherapy of early onset idiopathic chronic pancreatitis: results with long-term follow-up. Gastrointest Endosc. 2002;55:488–93.
- Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–9.
- Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–54.
- Mann ST, Stracke H, Lange U, Klor HU, Teichmann J. Alterations of bone mineral density and bone metabolism in patients with various grades of chronic pancreatitis. Metabolism. 2003;52:579–85.
- Duggan SN, Smyth ND, Murphy A, Macnaughton D, O’Keefe SJ, Conlon KC. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:219–28.
- Bernstein CN, Leslie WD. The pathophysiology of bone disease in gastrointestinal disease. Eur J Gastroenterol Hepatol. 2003;15:857–64.
- Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–94.
- Tignor AS, Wu BU, Whitlock TL, Lopez R, Repas K, Banks PA, et al. High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol. 2010;105:2680–6.
- Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Hamada S, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–9.
- Hanck C, Rossol S, Hartmann A, Singer MV. Cytokine gene expression in peripheral blood mononuclear cells reflects a systemic immune response in alcoholic chronic pancreatitis. Int J Pancreatol. 1999;26:137–45.
- Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int. 2011;22:435–46.
|
|
|
 |
|
|