|
|
|
|
 |
 |
| |
 |
|
|
Case Report |
|
|
|
|
|
Keywords :
|
|
|
amlodipin teva bivirkninger amlodipin sandoz
Department of Medical gastroenterology,
SSB – 3, Super Specialty Block 3rd Floor,
Govt. Medical College, Thiruvananthapuram 695011,
Kerala, India
Corresponding Author:
Dr Sojan George K
Email: sgkunnathil@gmail.com
DOI:
http://dx.doi.org/10.7869/tg.124
48uep6bbphidvals|609 48uep6bbph|2000F98CTab_Articles|Fulltext Strongyloides stercoralis is an intestinal nematode that affects more than 100 million people worldwide especially in tropical regions such as Southeast Asia, South America and sub- Saharan Africa. Gastrointestinal infestation may vary from asymptomatic to ileus or intestinal obstruction in patients with heavy intestinal infestation. Though Strongyloides infection can occur in immunocompetent adults, a search for an underlying immunosupression may result in early diagnosis and institution of therapy in diseases such as lymphoma[1]. On the other hand, a few studies have shown that Strongyloides hyperinfection may result in the development of adult T-cell leukaemia/lymphoma[2-4]. We report two patients of Strongyloides infection with different modes of gastrointestinal presentation, who later developed T cell lymphoma while on follow-up.
Case report 1
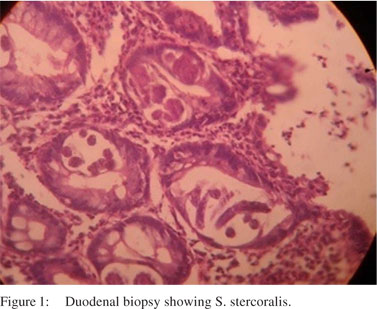
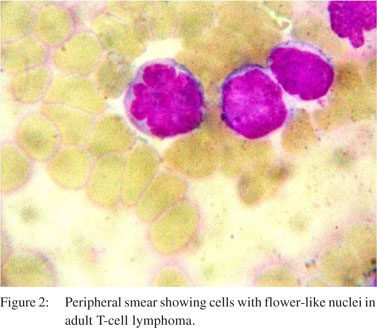
A 56-year-old man presented with chronic, large volume watery diarrhoea of 3 months’ duration. There was no blood or mucus in the stools. He had pedal oedema and a weight loss of 4 kg during this period. There was no past history of medical or surgical illness. On clinical examination he had pedal edema. He had no lymphadenopathy or visceromegaly. Laboratory investigations showed a leukocyte count of 11400 cells/mm³ with 8% eosinophils. His total serum protein level was 4.3 g/dL with an albumin level of 1.8 g/dL. Other biochemical investigations were normal and enzyme-linked immunosorbent array (ELISA) test for human immunodeficiency virus (HIV) was negative. His duodenal biopsy showed S. stercoralis (Figure 1). He was treated with four doses of 12 mg ivermectin on days 0, 1, 15 and 16; following which he improved symptomatically. Thirteen months later, he presented with generalized lymphadenopathy of one month duration and altered sensorium of one week duration. His clinical examination revealed enlarged cervical, submandibular, axillary and inguinal lymph nodes of sizes varying from 2–3 cm in diameter. He also had pedal oedema and hepatosplenomegaly. Laboratory investigation showed: haemoglobin level of 13.6 g/dL, leukocyte count of 62000 cells/mm³ (63% lymphocytes), erythrocyte sedimentation rate of 48 mm/hour, alkaline phosphatase levels of 784 U/L and serum albumin levels of 3 g/ dL. Peripheral smear showed atypical cells with irregularly cleaved nuclei and occasional cells with flower-like nuclei. Lymph node biopsy was consistent with adult T-cell lymphoma (Figure 2). The patient’s condition deteriorated after 24 hours of admission and he succumbed to cardiorespiratory failure.
 Case report 2
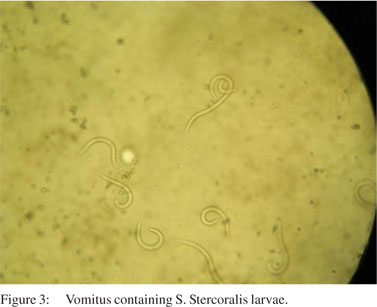
A 48-year-old woman presented with periumblical, colicky, postprandial abdominal pain of 8 months’ duration, with bilious vomiting and constipation of 2 weeks’ duration. She had a history of impaired fasting glucose detected recently and was advised dietary modification. Her clinical examination revealed a body mass index of 17.2 kg/m² with pallor, glossitis, cheilitis and tinea versicolor. She had a firm hepatomegaly. Laboratory investigations showed peripheral eosinophilia, erythrocyte sedimentation rate of 92 mm/hour, hypochromic microcytic anaemia in peripheral smear, and hypoalbuminaemia (2.7 g/dL). Rest of her laboratory investigations and ELISA test for HIV were negative. Contrast-enhanced computed tomography (CECT) scan showed minimal circumferential, irregular wall thickening in the junction of the second and third part of the duodenum with mild luminal narrowing. Her upper gastrointestinal endoscopy showed granular whitish appearance of the duodenum with narrowing at the periampullary region. Duodenal biopsy showed chronic duodenitis and mild tissue eosinophila with numerous strongyloides larvae. Her stool and gastric aspirate also showed strongyloides larvae (Figure 3). Bone marrow examination done to rule out any indolent malignancy was normal. She was treated with 12 mg ivermectin for 2 consecutive days repeated after 2 weeks. She improved with no further episodes of vomiting and was kept on follow-up. Six months later, she presented with abdominal pain and nausea. On examination, she was found to have generalized lymphadenopathy with enlarged cervical, axillary and intra-abdominal lymph nodes. Stool microscopy and duodenal aspirate was negative for strongyloides. Lymph node biopsy revealed adult T-cell lymphoma and she was started on chemotherapy.
Case report 2
A 48-year-old woman presented with periumblical, colicky, postprandial abdominal pain of 8 months’ duration, with bilious vomiting and constipation of 2 weeks’ duration. She had a history of impaired fasting glucose detected recently and was advised dietary modification. Her clinical examination revealed a body mass index of 17.2 kg/m² with pallor, glossitis, cheilitis and tinea versicolor. She had a firm hepatomegaly. Laboratory investigations showed peripheral eosinophilia, erythrocyte sedimentation rate of 92 mm/hour, hypochromic microcytic anaemia in peripheral smear, and hypoalbuminaemia (2.7 g/dL). Rest of her laboratory investigations and ELISA test for HIV were negative. Contrast-enhanced computed tomography (CECT) scan showed minimal circumferential, irregular wall thickening in the junction of the second and third part of the duodenum with mild luminal narrowing. Her upper gastrointestinal endoscopy showed granular whitish appearance of the duodenum with narrowing at the periampullary region. Duodenal biopsy showed chronic duodenitis and mild tissue eosinophila with numerous strongyloides larvae. Her stool and gastric aspirate also showed strongyloides larvae (Figure 3). Bone marrow examination done to rule out any indolent malignancy was normal. She was treated with 12 mg ivermectin for 2 consecutive days repeated after 2 weeks. She improved with no further episodes of vomiting and was kept on follow-up. Six months later, she presented with abdominal pain and nausea. On examination, she was found to have generalized lymphadenopathy with enlarged cervical, axillary and intra-abdominal lymph nodes. Stool microscopy and duodenal aspirate was negative for strongyloides. Lymph node biopsy revealed adult T-cell lymphoma and she was started on chemotherapy.
 Discussion
S. stercoralis is unique among intestinal nematodes in its ability to complete its life cycle within the host through an asexual autoinfectious cycle. The autoinfection cycle is normally prevented by the host immune system, most notably by the Th2 response. S. stercoralis is capable of transforming into a fulminant illness especially when there are defects in the cellmediated immunity.1 Steroid therapy and human T-lymphotropic virus-1 human T-lymphotropic virus-1 ((HTLV-1) infection are the most consistent associations. The altered cytokine milieu in cases of lymphoreticular and haematological malignancies may explain how these conditions result in S. stercoralis hyperinfection. Although acquired immunodeficiency syndrome (AIDS) and malnutrition have both been associated with hyperinfection, the number of reported cases is much less than expected.
The association between adult T-cell lymphoma and strongyloidiasis has been reported in the world literature.[2–4] In both the cases described above, the patients presented with T-cell lymphoma after one year of strongyloidiasis. Patients co-infected with HTLV-I and S. stercoralis have higher levels of interferon (IFN)-gamma and lower levels of interleukin (IL-5) and IL-13. This leads to a decreased S. stercoralis-specific type 2 immune response.[5] Clinically, patients coinfected with HTLVI and S. stercoralis may develop severe strongyloidiasis, as well as a low cure rate in response to anti-S. stercoralis drugs. On the other hand, some studies have shown that Strongyloides hyperinfection may result in the development of adult T-cell leukaemia/lymphoma. This may be due to a Strongyloides antigen that induces a potent polyclonal T cell mitogenic response, and reactivation of HTLV-1 expression.[6,7] The alternative hypothesis is that Strongyloides hyperinfection causes general immune suppression that permits HTLV-1 replication and spread. Expansion of infected T-cells may enhance genetic instability resulting in inactivation of DNA repair mechanisms, predisposing to cancer.[8,9] On the basis of the above studies and our case series, we recommend a close follow-up of patients with Strongyloides hyperinfection with an initial HTLV-1 serology, and a detailed clinical examination and haemogram every 3 months after the diagnosis.
References
Discussion
S. stercoralis is unique among intestinal nematodes in its ability to complete its life cycle within the host through an asexual autoinfectious cycle. The autoinfection cycle is normally prevented by the host immune system, most notably by the Th2 response. S. stercoralis is capable of transforming into a fulminant illness especially when there are defects in the cellmediated immunity.1 Steroid therapy and human T-lymphotropic virus-1 human T-lymphotropic virus-1 ((HTLV-1) infection are the most consistent associations. The altered cytokine milieu in cases of lymphoreticular and haematological malignancies may explain how these conditions result in S. stercoralis hyperinfection. Although acquired immunodeficiency syndrome (AIDS) and malnutrition have both been associated with hyperinfection, the number of reported cases is much less than expected.
The association between adult T-cell lymphoma and strongyloidiasis has been reported in the world literature.[2–4] In both the cases described above, the patients presented with T-cell lymphoma after one year of strongyloidiasis. Patients co-infected with HTLV-I and S. stercoralis have higher levels of interferon (IFN)-gamma and lower levels of interleukin (IL-5) and IL-13. This leads to a decreased S. stercoralis-specific type 2 immune response.[5] Clinically, patients coinfected with HTLVI and S. stercoralis may develop severe strongyloidiasis, as well as a low cure rate in response to anti-S. stercoralis drugs. On the other hand, some studies have shown that Strongyloides hyperinfection may result in the development of adult T-cell leukaemia/lymphoma. This may be due to a Strongyloides antigen that induces a potent polyclonal T cell mitogenic response, and reactivation of HTLV-1 expression.[6,7] The alternative hypothesis is that Strongyloides hyperinfection causes general immune suppression that permits HTLV-1 replication and spread. Expansion of infected T-cells may enhance genetic instability resulting in inactivation of DNA repair mechanisms, predisposing to cancer.[8,9] On the basis of the above studies and our case series, we recommend a close follow-up of patients with Strongyloides hyperinfection with an initial HTLV-1 serology, and a detailed clinical examination and haemogram every 3 months after the diagnosis.
References
- Keiser PB, Nutman TB. Strongloides stercoralis in the immunocompromised population. Clinical Microbiol Rev. 2004;17:208–17.
- Stewart DM, Ramanathan R, Mahanty S, Fedorko DP, Janik JE, Morris JC. Disseminated Strongyloides stercoralis infection in HTLV-1-associated adult T-cell leukemia/lymphoma. Acta Haematol. 2011;126:63–7.
- López, G, Gonzalez E, Clark D, Talledo M, Alvarez C, Verdonck K, et al. Proviral load does not discriminate patients with human T-cell leukemia/lymphoma (ATLL) from HTLV-1 carriers with a history of Strongyloidiasis. Retrovirology. 2011;8:A253.
- Matutes E. Adult T-cell leukaemia/lymphoma. J Clin Pathol. 2007;60:373–7.
- Porto AF, Neva FA, Bittencourt H, Lisboa W, Thompson R, Alcantara L, et al. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol. 2001;23:503–7.
- Gabet AS, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–60.
- Satoh M, Toma H, Sugahara K, Etoh K, Shiroma Y, Kiyuna S, et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)CD25(+) HTLV-1- infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene. 2002;21:2466–75.
- Park HU, Jeong JH, Chung JH, Brady JN. Human T-cell leukemia virus type 1 Tax interacts with Chk1 and attenuates DNA-damage induced G2 arrest mediated by Chk1. Oncogene. 2004;23:4966–74.
- Franchini G, Nicot C, Johnson JM. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv Cancer Res. 2003;89:69–132.
|
|
|
 |
|
|