Sudeep Banerjee1, Jennifer Premkumar2, Marie Therese Manipadam2, Anu Eapen3, Philip Joseph1, Frederick Lorence Vyas1, Ravish SanghI Raju1, Venkatramani Sitaram1
Division of Gastrointestinal Surgery1 (HPB Unit),
Departments of Pathology2 and Radiodiagnosis3
Christian Medical College, Vellore-632004, Tamil Nadu, India.
Corresponding Author:
Dr. Venkatramani Sitaram
Email: sitaram@cmcvellore.ac.in; sur4@cmcvellore.ac.in
DOI:
http://dx.doi.org/10.7869/tg.127
48uep6bbphidvals|612 48uep6bbph|2000F98CTab_Articles|Fulltext Perivascular epithelioid cell tumour (PEComa) is a rare neoplasm. It is composed chiefly of HMB-45-positive epithelioid cells and is rich in vascular organoid architecture.
Case report
A 25-year-old man presented with melaena for 4 days. There was no history of abdominal pain, vomiting, jaundice or altered bowel habit. He was pale. Other physical examinations were normal. Laboratory investigations included packed cell volume 23%, marginally high C-reactive protein (30.9 mg/L), normal coagulation profile and liver function tests (LFTs).
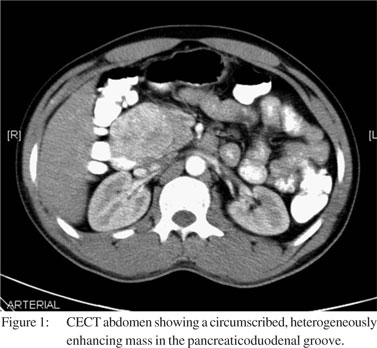
Oesophagogastroduodenoscopy showed nodular, ulcerated and friable mucosa in the proximal second part of the duodenum, suggestive of infiltration. Duodenal mucosal biopsy was reported as spindle cell proliferation, with the possibility of an underlying smooth muscle tumour. The tumour cells had displayed positive staining for smooth muscle actin (SMA), and occasional cells were positive for CD 117, S100 and CD34. Contrast-enhanced CT (CECT) scan showed a 7 cm × 5 cm, heterogeneously enhancing mass in the pancreaticoduodenal groove with thickening of the duodenal wall (Figure 1). The mass displaced the pancreatic head without obvious infiltration. The patient underwent pancreaticoduodenectomy with radiological access loop creation for hepaticojejunostomy. A solid cystic mass was seen in the region of the pancreatic head during the operation.
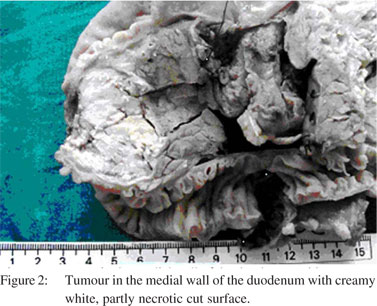
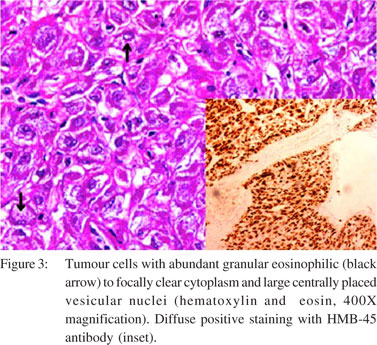
Grossly, it was a 5 cm circumscribed tumour in the wall of the duodenum, at the level of the papilla. There was no invasion of the adjacent pancreas. The lesion had a creamy white, partly necrotic cut surface (Figure 2). Microscopically, the lesion was found to originate in the submucosal layer of the duodenal wall with areas of mucosal ulceration and necrosis. Tumour cells were monomorphic, arranged as lobules and sheets of large polygonal cells with abundant granular eosinophilic to focally clear cytoplasm and large centrally placed vesicular nuclei (Figure 3). Prominent central eosinophilic nucleoli were present in the foci. The lobules were separated by sinusoidal vessels. The tumour was demarcated from the pancreas by fibrous capsule and there were foci of calcification. Lymph nodes were reactive. Mitotic activity was 1 in 50 high power fields. The phenotype of tumour cell was verified by immunohistochemistry. Cells were diffusely positive for HMB- 45 antibody (melanocytic marker) (Figure 3 inset), and stained equivocally for neuron-specific enolase (NSE). Scattered tumour cells were positive for desmin and SMA.
Immunostaining for CD34, S100, synaptophysin, CD117, cytokeratin, alpha-foetoprotein (AFP) and placental alkaline phosphatase (PLAP) were negative. The pathological features were consistent with PEComa. The patient was asymptomatic with normal CT scan and LFT at 6 months of follow-up.
 Discussion
PEComa is composed of perivascular epithelioid cells (PECs) with myomelanocytic differentiation and strong immunoreactivity for HMB-45 antibody. It is a rare but discretely-defined neoplasm.[1] These tumours may arise in any anatomical location as PECs do not have a normal anatomic homologue. PEComa and related tumours of the duodenum are exceptionally rare, with previously reported literature limited to three case reports—one paediatric patient with PEComa and two adult patients with angiomyolipoma.[2–4] Gastrointestinal PEComa shows a marked preponderance in women and onethird of the cases occur in the paediatric age group.[1] These tumours may be associated with neuroblastoma and tuberous sclerosis.[2,5]
PEComa seems to have a similar biological behaviour to GIST.[6] The negative reactivity for CD117 and CD34 went against the diagnosis of epithelioid GIST and KIT-negative GIST. Endocrine tumours are usually positive for NSE and synaptophysin. Cytokeratin was negative, excluding the diagnosis of carcinoma. Germ cell tumour was ruled out by the negative reaction for AFP and PLAP. Malignant melanoma and granular cell tumour could not be considered because S100 was negative. Smooth muscle tumour with epithelioid morphology was considered as a differential diagnosis due to the positivity of few cells for desmin and SMA. However, there were no spindled foci with perinuclear vacuoles or cigarshaped nuclei. In our patient, the morphology of the tumour showed abundant granular eosinophilic to focally clear cytoplasm, delicate sinusoidal network and presence of cytoplasmic glycogen; these were in favour of PEComa.[7] The immunohistochemical reaction with diffuse strong positivity for HMB-45 antibody, and focal positive staining for SMA, highlighted the myomelanocytic nature of this tumour.[5,7]
Discussion
PEComa is composed of perivascular epithelioid cells (PECs) with myomelanocytic differentiation and strong immunoreactivity for HMB-45 antibody. It is a rare but discretely-defined neoplasm.[1] These tumours may arise in any anatomical location as PECs do not have a normal anatomic homologue. PEComa and related tumours of the duodenum are exceptionally rare, with previously reported literature limited to three case reports—one paediatric patient with PEComa and two adult patients with angiomyolipoma.[2–4] Gastrointestinal PEComa shows a marked preponderance in women and onethird of the cases occur in the paediatric age group.[1] These tumours may be associated with neuroblastoma and tuberous sclerosis.[2,5]
PEComa seems to have a similar biological behaviour to GIST.[6] The negative reactivity for CD117 and CD34 went against the diagnosis of epithelioid GIST and KIT-negative GIST. Endocrine tumours are usually positive for NSE and synaptophysin. Cytokeratin was negative, excluding the diagnosis of carcinoma. Germ cell tumour was ruled out by the negative reaction for AFP and PLAP. Malignant melanoma and granular cell tumour could not be considered because S100 was negative. Smooth muscle tumour with epithelioid morphology was considered as a differential diagnosis due to the positivity of few cells for desmin and SMA. However, there were no spindled foci with perinuclear vacuoles or cigarshaped nuclei. In our patient, the morphology of the tumour showed abundant granular eosinophilic to focally clear cytoplasm, delicate sinusoidal network and presence of cytoplasmic glycogen; these were in favour of PEComa.[7] The immunohistochemical reaction with diffuse strong positivity for HMB-45 antibody, and focal positive staining for SMA, highlighted the myomelanocytic nature of this tumour.[5,7]
References
- Ryan P, Nguyen VH, Gholoum S, Carpineta L, Abish S, Ahmed NN, et al. Polypoid PEComa in the rectum of a 15-year-old girl: case report and review of PEComa in the gastrointestinal tract. Am J Surg Pathol. 2009;33:475–82.
- Mhanna T, Ranchere-Vince D, Hervieu V, Tardieu D, Scoazec JY, Partensky C. Clear cell myomelanocytic tumor (PEComa) of the duodenum in a child with a history of neuroblastoma. Arch Pathol Lab Med. 2005;129:1484–6.
- De Padua M, Gupta N, Broor SL, Govil D. Duodenal angiomyolipoma: a case report. Indian J Pathol Microbiol.2007;50:568–9.
- Toye LR, Czarnecki LA. CT of a duodenal angiomyolipoma. Am J Roentgenol. 2002;178:92.
- Vang R, Kempson RL. Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: a subset of HMB-45 positive epithelioid mesenchymal neoplasm with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26:1–13.
- Birkhaeuser F, Ackermann C, Flueckiger T, Guenin MO, Kern B, Tondelli P, et al. First description of a PEComa (perivascular epithelioid cell tumor) of the colon: report of a case and review of the literature. Dis Colon Rectum. 2004;47:1734–7.
- Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82.
|