|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
Cytokines, IL-6, TNF- a, IL-10, IBS |
|
|
Rana SV, Sharma S, Sinha SK, Parsad KK, Malik A, Singh K
Department of Gastroenterology,
Postgraduate Institute of Medical
Education and Research,
Sector-12, Chandigarh, India
Corresponding Author:
Dr. SV Rana
Email: svrana25@hotmail.com
DOI:
http://dx.doi.org/10.7869/tg.2012.66
Abstract
Background and aim: Irritable bowel syndrome (IBS) is referred to as a functional bowel disorder which is diagnosed by a number of characteristic symptoms (Rome II criteria) in the absence of detectable structural abnormalities. Low-grade inflammation of the intestine may be one of the reasons for development of diarrhoea-predominant IBS (IBS-D). We undertook this study to estimate the serum levels of pro-inflammatory (IL-6, TNF-a) and anti-inflammatory (IL-10) cytokines in IBS-D patients.
Methods: A total of 108 diarrhoea patients were screened. Out of these only 63 adult IBS-D patients were enrolled. Age and sex matched 62 apparently healthy controls with no GI symptoms were also recruited. Out of 63 IBS-D patients, 37 were males while there were 32 males among the controls. The patients with IBS-D were diagnosed according to the Rome II criteria. Levels of serum IL-6, TNF-a and IL-10 were measured in all subjects using ELISA. Results: Mean (+SD) age of IBS-D patients (42.6+19.5 years) was comparable (p=0.64) to that of controls (43.5+18.7 years). The mean (+SD) levels of IL-6 in IBS-D patients (32.2+12.01pg/ ml) was significantly higher (p<0.001) than in controls (7.48+2.55pg/ml). The levels of TNF-a in IBS-D patients (16.3+5.2 pg/ml) were also significantly higher (p<0.05) than in controls (7.94+2.19 pg/ml). There was no significant difference in the serum levels of IL-10 (p=0.23) between IBS-D patients (5.75+2.1 pg/ml) and controls (5.84+1.9 pg/ml).
Conclusion: Our results indicate that mild inflammation is involved in IBS-D patients as proinflammatory
cytokines were increased although no difference in anti-inflammatory cytokine was observed.
|
48uep6bbphidvals|551 48uep6bbph|2000F98CTab_Articles|Fulltext Irritable bowel syndrome is a common functional bowel disorder characterized by recurrent abdominal pain and altered bowel habits.[1] The condition is common, with a population prevalence of between 5 and 20%.[2-4] Several mechanisms have been proposed to explain the pathophysiology of IBS, including visceral hypersensitivity,[5] altered gut motility,[6] and psychosocial factors.[7] In addition, inflammation and mucosal immune system activation may be important.[8] Studies demonstrate an increased risk for developing IBS after dysenteric illness[9-11] and increased numbers of immunocompetent cells in rectal mucosa of patients with postinfectious IBS up to 1 year,[12] implying that low-grade inflammation may contribute to symptoms. In a recent review it has been suggested that low-grade mucosal inflammation, particularly mast cell activation, may be a contributory factor in the pathogenesis of IBS.[13] Pro- and anti-inflammatory cytokines are important modulators of the immune response and play a role in intestinal inflammation. The normal gastrointestinal immune response is under tight regulation with the balance between pro- and anti-inflammatory cytokines/mediators defining the immune status of the gut.[14]
IL-6 is a multi-functional cytokine that regulates immune response, acute phase reactions, hematopoiesis and may play a central role in host defense mechanisms.[15] This cytokine exerts many effects ranging from defense to inflammation and tissue damage.[16] It is produced both by macrophages and adipocytes,[17] and by immune cells, fibroblasts and endothelial cells.[18]
Tumor necrosis factor alpha (TNFa) is a polypeptide cytokine produced by monocytes and macrophages which has a crucial role in chronic inflammatory states such as inflammatory bowel disease[17,20] and rheumatoid arthritis.[21] It has been shown that patients with persistent symptoms after an acute infectious gastroenteritis have a five-fold increase in the number of activated macrophages in the rectal lamina propria.[13] TNFa circulates throughout the body responding to stimuli (infectious agents or tissue injury), activating neutrophils, altering the properties of vascular endothelial cells, regulating metabolic activities of other tissues, as well as exhibiting tumoricidal activity by inducing localized blood clotting. Due to its varied actions throughout the immune system, TNF-a may play a role in the pathogenesis of many disease states.[22] Macrophages are the main producers of TNF- a and interestingly are also highly responsive to TNF-a. TNF- a has been shown to play a pivotal role in orchestrating the cytokine cascade in many inflammatory diseases and because of its role as a “master-regulator” of inflammatory cytokine production.[23]
Interleukin 10 is a pleiotropic cytokine playing an important role as a regulator of lymphoid and myeloid cell function. Due to its ability to block cytokine synthesis and several accessory cell functions of macrophages, this cytokine is a potent suppressor of the effector functions of macrophages, T-cells and NK cells. In addition, IL-10 participates in regulating proliferation and differentiation of B-cells, mast cells and thymocytes.[24] The immunosuppressive properties of IL-10 suggest a possible clinical use in suppressing transplant graft rejections. IL-10 can furthermore exert strong anti-inflammatory activities.[25]
Therefore, we hypothesize that low-grade inflammation of the intestine may be the reason for development of IBS-D. This study was planned to estimate serum levels of proinflammatory cytokines (IL-6, TNF-a) and anti-inflammatory cytokine (IL-10) in adult patients of diarrhoeal type of IBS (IBS-D).
Methods
Patients were diagnosed as per the Rome II criteria.[26] IBS is diagnosed in the presence of abdominal pain or discomfort for at least 12 weeks in the preceding 12 months associated with two of the following: relief after a bowel movement and / or association with a change in frequency of bowel movements; and / or an association with a change in consistency of motions. All IBS subjects were further classified according to the Rome II criteria for bowel habit predominance and only diarrhoea predominant IBS (IBS-D) patients were included for this study. A total of 108 patients suffering from diarrhoea were screened.
Out of these only 63 adult IBS-D patients fulfilled the Rome II criteria. Age and sex matched 62 apparently healthy individuals with no GI symptoms were recruited as controls. Biopsies were taken by sigmoidoscopy in order to exclude microscopic colitis in all patients. The study was approved by our Institute ethics committee and informed consent was obtained from all subjects before their enrollment. Patients with other gastrointestinal diseases including inflammatory bowel disease (IBD) confirmed by sigmoidoscopy, and clinically significant systemic diseases were excluded from the study. Pregnant women, individuals diagnosed with lactose intolerance, immunodeficiency, individuals who had undergone any abdominal surgery, with the exception of hernia repair and appendectomy, and those with a psychiatric illness were also excluded.
Cytokine assays
Peripheral venous blood samples were collected from all participants. Five ml blood samples were collected in plain vials. Samples were centrifuged after proper clotting to prevent hemolysis. Serum was separated and stored at -80°C until further analysis. Serum IL-6, TNF-a and IL-10 were measured by a commercially available enzyme-linked immunosorbent assay (Diaclone). IBS-D patients and controls samples were processed in same run for proper comparison. The assays were performed according to the manufacturer’s protocols. The minimal detectable concentration were <2 pg/ml, <3.16 pg/ml, <1.3 pg/ ml for IL-6, TNF-a and IL-10, respectively. Inter- and intraassay assessments of the kit reliability were also conducted.
Statistical analysis
Statistical significance for serum cytokine levels between IBSD patients and healthy controls was evaluated using an unpaired two-tailed Student’s t-test. Values have been reported as means+SD. Any statistical difference was considered significant at p <0.05.
Results
Out of the 108 patients screened, 45 were excluded (21 patients with IBS-A / M, five positive for microcytic colitis ruled out by sigmoidoscopy biopsy, eight positive for celiac diseases confirmed by serum TTG levels, seven patients positive for ova/cyst in stool and four positive for Clostridium difficile toxin in stool). Therefore, only 63 IBS-D patients were enrolled according to the Rome II criteria.[26]
Subject characteristics
The demographic data of patients and controls is given in Table 1. Mean (+SD) age of IBS-D patients (42.6+19.5 years) was comparable (p=0.64) to controls (43.5+18.7 years). Among the IBS-D patients 37 were males and 26 females while in controls 32 were males and 30 were females. Age and sex distribution among IBS-D patients and controls was found to be comparable. The age range in IBS-D patients was 26-65 years while that in controls was 25-64 years.
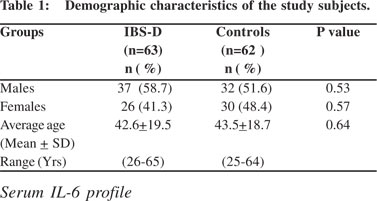 Figure 1 shows the scatter diagram of serum IL-6 cytokine profile for IBS-D patients and healthy controls. There was a significant difference (p<0.001) between the IL-6 serum levels of IBS-D patients (32.2+12.01 pg/ml) and healthy controls (7.48+2.55 pg/ml).
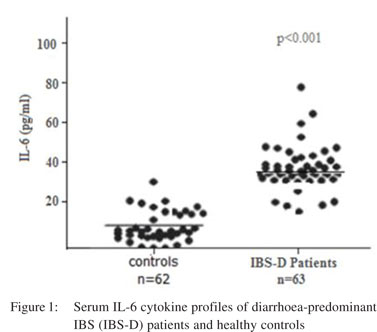
Serum TNF-a profile
Figure 2 shows the cytokine profile of IBS-D patients and healthy controls. There was a significant difference (p<0.05) in serum levels of TNF-a between IBS-D patients and healthy controls (16.3+5.2 vs. 7.94+2.19 pg/ml).
Figure 1 shows the scatter diagram of serum IL-6 cytokine profile for IBS-D patients and healthy controls. There was a significant difference (p<0.001) between the IL-6 serum levels of IBS-D patients (32.2+12.01 pg/ml) and healthy controls (7.48+2.55 pg/ml).
Serum TNF-a profile
Figure 2 shows the cytokine profile of IBS-D patients and healthy controls. There was a significant difference (p<0.05) in serum levels of TNF-a between IBS-D patients and healthy controls (16.3+5.2 vs. 7.94+2.19 pg/ml).
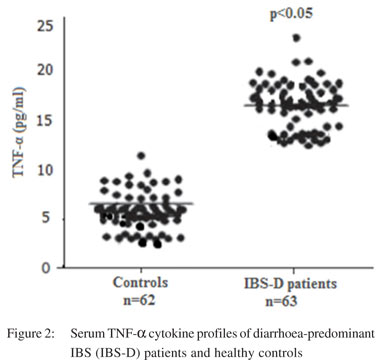
Serum IL-10 profiles
Figure 3 shows the IL-10 cytokine profile observed in IBS-D patients and healthy controls. No significant difference (p=0.23) was observed between the serum levels of IL-10 (5.75+2.1 pg/ ml) in IBS-D patients and the healthy controls (5.84+1.9 pg/ml). Correlation between pro-inflammatory cytokine levels and severity of symptoms
No correlation was noted between pro-inflammatory cytokine levels and severity of symptoms. Values of correlation were as follows: IL-6 vs. stool frequency (r=0.42, p=0.14); IL-6 vs. Bristol stool scores (r=0.32, p=0.17); IL-6 vs. abdominal pain (r=0.45, p=0.15); IL-6 vs. bloating scores (r=0.35, p=0.42); TNF-a vs. stool frequency (r=0.32, p=0.21); TNF-a vs. Bristol stool scores (r=0.35, p=0.19); TNF-a vs. abdominal pain (r=0.38, p=0.18); TNF-a vs. bloating scores (r=0.39, p=0.22).
Discussion
Irritable bowel syndrome is a stress-related disorder associated with disturbed brain-gut communication, gastrointestinal homeostasis and based on recent evidence, low-grade inflammation and an altered microbiota. Our study examines the cytokine profiles of IBS-D patients and healthy individuals.
The presence of elevated serum IL-6 and TNF-a in diarrheapredominant IBS patients as compared to controls signifies ongoing mild inflammation in IBS-D. Our findings are consistent with a recent study by Scully et al.[27] Their analysis of proinflammatory cytokines in female IBS patients with extraintestinal co-morbidities showed increased levels of IL-6, IL-8, and TNF-a. McKerman et al[28] also demonstrated elevated cytokine levels in patients with the irritable bowel syndrome, indicating some immune dysregulation in these patients. Proinflammatory cytokines IL-6 and TNF-a have been shown to control the release of corticotrophin-releasing hormone, the main hypothalamic regulatory peptide of the hypothalamicpituitary- adrenal (HPA) axis.[29,30-31] Thus an increase in these cytokines as seen in our IBS patients may be involved in exaggerated activation of the HPA axis. The significant increase in IL-6 and TNF-a levels in our IBS-D patients tend to corroborate with the above hypothesis. Similar findings of increased serum IL-6 and TNF-a in IBS patients have been reported in other studies.[29,32,33] This may be due to enhanced cellular immune response with increased pro-inflammatory cytokine production in diarrhea-predominant IBS. It has further been seen that IL-6 and TNF- a cytokine gene polymorphisms could change an individual’s susceptibility to IBS and these might have pathophysiological role.[34] However, no differences were observed in TNF-a and IL-6 levels between patient groups with strong and mild/moderate pain intensity.[35] It has also been observed by Dinan et al[36] that the IL-6 cytokine profile in IBS is driven by responses mediated by muscarinic receptors. IL-10 is a potent suppressor of macrophage, T cell and NK cell effector functions. In addition, IL-10 participates in regulating proliferation and differentiation of B-cells.[24] We did not find any significant difference in the IL-10 levels between our patients and controls. The ongoing mild inflammation discussed above might be the reason for such observations for IL-10. These findings are similar to those reported by other studies.[27,29] IL-10 has also been proposed to have a putative role in enteric infections and other causes of intestinal inflammation. High producer TNF-a and low producer IL-10 genotypes have been reported to be significantly more prevalent in IBS patient than in healthy individuals and also in diarrhea-predominant IBS as compared to other IBS subtypes.[37]
The lower prevalence of the high producer genotype in IBS suggests that high production of IL-10 may have some protective role or, conversely, that individuals predisposed to produce lower amounts of this cytokine might be more likely to develop IBS.[38] Studies have documented the onset of IBS following bacteriologically confirmed gastroenteritis, while others have provided evidence of low-grade mucosal inflammation[12,39] and immune activation[40,41] in IBS. Mast cells, which are increased in number and activated in the jejunum and colon in IBS, may play a critical role in mediating the neural response to these inflammatory triggers. The results by Dinan et al[42] show that mast cells increased significantly in the IBSdiarrhea group compared to IBS-constipation and control groups. Despite the expected heterogeneity of the disorder, differences in mucosal chemokine signaling were evident in this cross-sectional study of IBS patients at the level of both gene expression and protein secretion.[43] It has also been reported that specific environment events such as acute gastrointestinal infection can trigger the manifestations of IBS.
Moreover, in animal studies the role of transient mucosal inflammation[44] and the role of the severity of inflammation in terms of the manifestation of specific abnormalities of function have been identified.[45] Thus it seems reasonable to speculate that inflammatory process can play an important role in the manifestations of IBS-D.
This study demonstrates that patients with IBS-D can have increased levels of pro-inflammatory cytokines IL-6 and TNF-a with no difference in anti-inflammatory cytokine levels of IL-10. Our data provides evidence to support the view that mild inflammation may be involved in diarrheal type of irritable bowel syndrome.
References
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F and Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91.
- Agreus L, Talley NJ, Svardsudd K, Tibblin G and Jones MP. Identifying dyspepsia and irritable bowel syndrome: the value of pain or discomfort, and bowel habit descriptors. Scand J Gastroenterol. 2000;35:142–51.
- Hillila MT and Farkkila MA. Prevalence of irritable bowel syndrome according to different diagnostic criteria in a nonselected adult population. Aliment Pharmacol Ther. 2004;20:339–45.
- Hungin AP, Whorwell PJ, Tack J and Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50.
- Mertz H, Naliboff B, Munakata J, Niazi N and Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52.
- Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy SN, Mena I and Snape WJ, Jr. Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology. 1991;101:1298–306.
- Whitehead WE and Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263–71.
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83.
- Neal KR, Hebden J and Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779–82.
- Rodriguez LA and Ruigomez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565–6.
- Wang LH, Fang XC and Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–101.
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11.
- Ford AC and Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–31.
- Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9.
- Hirano T, Kishimoto T. Interleukin-6. In: Handbook of experimental pharmacology. Peptide growth factors and their receptors. Edited by Sporn MB, Roberts AB. Berlin. Springer- Verlag.1990:633–65.
- Fonseca-Alaniz MH, Takada J, Alonso-Vale MI and Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J). 2007;83:S192–203.
- Cachofeiro V, Miana M, Martin B. Obesidad inflamacion disfuncion endothelial. Rev Esp Obes. 2006;5:195–204.
- Sanchez F, Garcia R, Alarcon F, Cruz M. Adipocinas, tejido adiposo y su relación con células del sistema inmune. Gac Med Mex. 2005;141:505–12.
- C Cayphas S, Van Damme J, Vink A, Simpson RJ, Billiau A and Van Snick J. Identification of an interleukin HP1-like plasmacytoma growth factor produced by L cells in response to viral infection. J Immunol. 1987;139:2965–9.
- Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O and Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–74.
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E and Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202.
- Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–43.
- Parameswaran N and Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103.
- Moore KW, O’Garra A, de Waal Malefyt R, Vieira P and Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90.
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG and de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20.
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl II):II43/II7.
- Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–43.
- McKernan DP, Gaszner G, Quigley EM, Cryan JF and Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045–52.
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–11.
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62.
- Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43:429–35.
- Schmulson M, Pulido-London D, Rodriguez O, Morales-Rochlin N, Martinez-Garcia R, Gutierrez-Ruiz MC, et al. Lower serum IL-10 is an independent predictor of IBS among volunteers in Mexico. Am J Gastroenterol. 2012;107:747–53.
- Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20.
- Barkhordari E, Rezaei N, Ansaripour B, Larki P, Alighardashi M, Ahmadi-Ashtiani HR, et al. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J Clin Immunol. 2010;30:74–9.
- Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL and Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52:376–81.
- Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, et al. Enhanced cholinergic-mediated increase in the proinflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–6.
- van der Veek PP, van den Berg M, de Kroon YE, Verspaget HW and Masclee AA. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–6.
- Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ and Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–3.
- Dunlop SP, Jenkins D, Neal KR and Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–9.
- Cumberland P, Sethi D, Roderick PJ, Wheeler JG, Cowden JM, Roberts JA, et al. The infectious intestinal disease study of England: a prospective evaluation of symptoms and health care use after an acute episode. Epidemiol Infect. 2003;130:453–60.
- Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–6.
- Goral V, Kucukoner M and Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology. 2010;57:751–4.
- Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–76.
- Liebregts T, Adam B, Bertel A, Jones S, Schulze J, Enders C, et al. Effect of E. coli Nissle 1917 on post-inflammatory visceral sensory function in a rat model. Neurogastroenterol Motil. 2005;17:410–4.
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–86.
|
|
|
 |
|
|