Gastric cancer (GC) is not uncommon and is a potentially fatal malignancy.
[1] H. pylori infection, dietary and host factors contribute to the development of GC.
[2,
3] Development of GC follows a multi-step process through chronic active gastritis (CAG), atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia, carcinoma-in-situ and finally early and advanced GC.
[4] GC is often diagnosed at an early stage in Japan, due to high level awareness and screening programs, resulting in better survival rates.[
5] In contrast, due to late diagnosis, prognosis of GC is poor in India. Since the frequency of GC is low in India, a screening program using an invasive tool like endoscopy for all subjects infected with H. pylori, is unlikely to be costeffective and practical.
Pepsinogen is the inactive precursor of gastric proteolytic enzyme pepsin.
[6] Human pepsinogen comprises of two isoenzymes, pepsinogen I (or A) and pepsinogen II (or C).
[7,
8] Pepsinogen I (PG-I) is secreted mainly by chief cells of fundic mucosa, whereas pepsinogen II (PG-II) is also secreted by pyloric glands and proximal duodenal mucosa.
[9,
10] While serum levels of PG-I correlate with levels of acid secretion, PG-II shows an inverse relationship.6 Therefore, the PG-I/PG-II ratio is a useful tool to evaluate gastric acid secretion, gastritis and intestinal metaplasia.
[11] Gastrin secretion is regulated by a negative feedback mechanism looped with acid secretion.
[12] It has been shown that serum PG-I, PG-II and G-17 levels are altered in atrophic gastritis and IM.
[13] Smoking and alcohol may also affect acid secretion, pepsinogen and G-17 levels.
[14,
15]
However, there is scanty data on utility of these serological markers in non-invasive screening for gastric pre-neoplastic lesions including IM from areas with high incidence of H. pylori infection but low risk of GC. The current study was therefore, designed to evaluate the utility of serum PG levels, their ratio and G-17 as non-invasive tools for diagnosis of IM and gastritis in a low incidence area of GC, endemic for H. pylori infection.
Methods
Patients
Diagnosis of GC was based on detection of typical lesions at upper gastrointestinal (UGI) endoscopy and by documentation of adenocarcinoma on histopathological examination of endoscopic biopsy.
[16] Patients with symptoms of dyspepsia in absence of peptic ulcer on UGI endoscopy were taken as controls.
[17]
Patients enrolled in the study had received no prior treatment for H. pylori infection and had not received proton pump inhibitors for a minimum of 4 weeks prior to study enrollment. Institutional ethics committee approved the study protocol; and informed consent was taken from all patients and control
prior to enrollment.
Histology
Four to six biopsies were obtained from suspicious lesions to diagnose GC and 4 were retrieved from normal looking areas away from the lesion(s). The specimen were collected in formalin and sent for gastritis scoring, H. pylori detection and IM on histopathology. In patients with dyspepsia, 4 biopsies were taken from the antrum and body in formalin for gastritis, H. pylori and IM detection.
Diagnosis of IM
IM was evaluated using haematoxylin and eosin (H & E), PAS and Alcian blue staining of sections according to a standard protocol (Figure 1).
[18]
Histological examination for scoring and topography of gastritis
Biopsies from areas away from the tumor were assessed for presence, grade (mild, moderate and severe) and topography of gastritis according to the updated Sydney system (1994).
[19]
This was assessed by two expert pathologists, who were blinded about the endoscopic findings and serum PG levels. When the scores between the two pathologists were different, the more severe score was selected.
Diagnosis of H. pylori infection
H. pylori infection was diagnosed by rapid urease test (RUT), histopathology and enzyme linked immunosorbent assay (ELISA) for HpIgG antibodies. Two biopsies, one each from antrum and body of the stomach were used for RUT at the time of endoscopy. A change in color from yellow to pink during a 2h observation period was considered positive for H. pylori infection.
[20] Serum level of anti- H. pylori IgG was estimated using a commercially available Biohit gastrosoft ELISA kit as per the manufacturer’s instruction.A cut-off value of antibody concentration >30 enzyme immunounit (EIU) was considered positive. Histopathological examination was undertaken using H&E and Giemsa stains. Positive results in any two of the three tests were considered to be diagnostic of H. pylori infection.
Smoking and alcohol consumption
A questionnaire was used to collect information on smoking (in form of cigarette, ‘hukka’, ‘bidi’), tobacco chewing (‘surti’, ‘gutkha’ and ‘pan’ with ‘jarda’) and alcohol consumption from all the subjects.
Serum PG-I, PG-II and gastrin-17
Twelve hour fasting blood was collected from all patients with GC and dyspepsia after informed consent. The samples were stored at -40°C till further use. Microplate-based quantitative immunoassay of serum PG-I, PG-II and G-17 were performed using commercially available ELISA kits (Biohit Oyj, Finland) as per manufacturer’s instructions. The standard curves were plotted to calculate final concentrations and results.
Statistical analysis
Patients were categorized on the basis of disease group, presence and absence of IM, H. pylori infection, gastritis status, smoking and alcohol consumption. Inter-group comparison between two continuous variables was performed using Mann-Whitney U test. Categorical variables were compared using Chi-square test with Yate’s correction as applicable. A p-value <0.05 was considered significant.
Receiver operating characteristic (ROC) curve was constructed for levels of PG-I, PG-II, PG-I/PG-II ratio and G-17 to determine the best cut–off values that differentiated between the presence and absence of IM on gastric biopsy, with highest sensitivity and specificity.
Results
Patients
A total of 98 patients with GC and 62 with dyspepsia were enrolled prospectively for this study, from September 2003 to December 2009, after detailed clinical evaluation.They were comparable in age (mean age +SD =54+11 years vs. 52+10 years, p=ns) and gender [74 (75.5%) vs. 41 (66.1%) males;
p=ns].
Results of histopathology for IM and gastritis
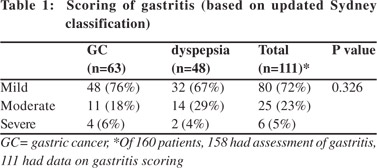
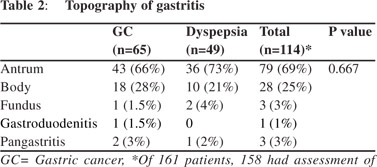
Patients with GC had a higher frequency of IM (35/98 [35%] vs. 9/62 [14%]; p = 0.006) and had higher grades of gastritis (Table 1) than those with dyspepsia. Of 160 patients (98 GC and 62 dyspepsia), 44/160 (27.5%) had IM and 112/158 (70.9%) had gastritis. Patients with H. pylori infection had higher rates of gastritis than those without (61 [54%] vs. 51 [45%]). Severity and topography of gastritis were similar among patients with GC and dyspepsia (Tables 1, 2).
H. pylori infection
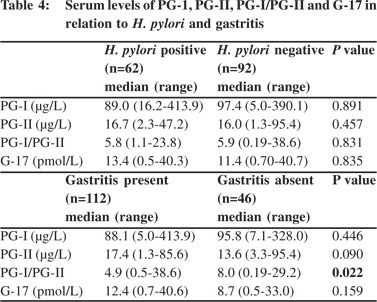
H. pylori was detected less frequently among patients with GC than dyspepsia (29/96 [30%] vs. 33/61 [54%]; p = 0.004) (Table 3). Serum levels of PG-I, PG-II, PG-I/PG-II and G-17 were comparable among H. pylori infected and non-infected patients (Table 4).
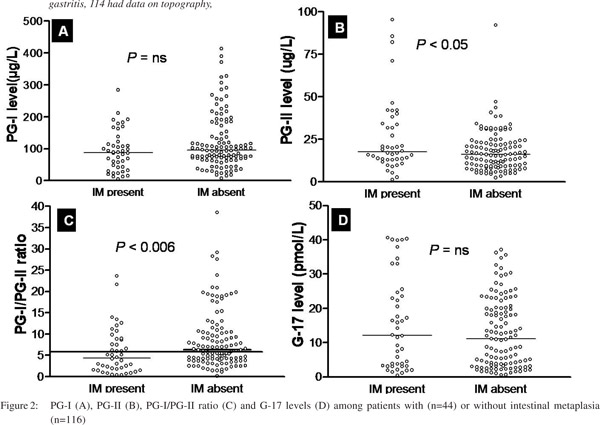
Comparison of serum levels of PG-I, PG-II, PG-I/PGII and G-17 in relation to IM Patients with IM had higher PG-II and lower PG-I/PG-II ratio than those without, though their serum PG-I levels were comparable (Table 5). The cut-off value of PG-I/PG-II ratio of 6.0 had a sensitivity of 64% and specificity of 52% for detection of IM (area under curve 0.64). 26/44 (60%) patients with IM and 52/98 (53%) with GC had PG-I/PG-II ratio <6. However, G- 17 levels were comparable among those with and without IM (Table 5, Figure 2).

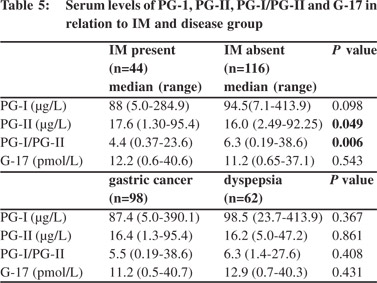 Relationship between PG levels, its ratio and gastritis
Relationship between PG levels, its ratio and gastritis
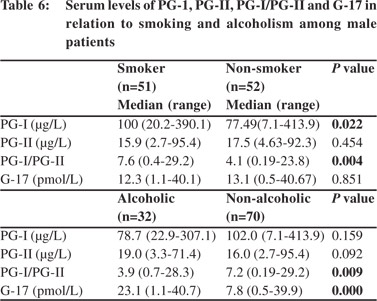
Patients with gastritis had lower PG-I/PG-II ratio (4.9 [0.5-38.6] vs. 8.0 [0.19-29.2], p = 0.02) than those without. A cut-off value of PG-I/PG-II ratio of 7 predicted gastritis with a sensitivity and specificity of 55% and 52%, respectively (area under curve 0.61). PG-I, PG-II and G-17 were similar amongst patients with and without gastritis (Table 4). PG levels and its ratio were also comparable among patients with different grades of gastritis (mild, moderate and severe). Comparison of serum levels of PG-I, PG-II, PG-I/PGII and G-17 in relation to smoking and alcohol consumption 51/141(36.2%) male patients were smokers and 32/141 (22.7%) were alcoholics. None of the female patients smoked or consumed alcohol. The smokers had higher PG-I and PG-I/PGII ratio than non-smokers, though PG-II and G-17 levels were comparable between both groups (Table 6).
The PG-I/PG-II ratio was lower and G-17 levels higher among alcoholics than non-alcoholics [3.9 (0.7-28.3) vs. 7.2 (0.19-29.2), p = 0.009 and 23.1 (1.1-40.7) vs. 7.8 (0.5-39.9), p= 0.001, respectively]. However, PG-I and PG-II levels were comparable among the two groups (Table 6).
Discussion
The aim of this study was to evaluate the utility of non-invasive serological tests to screen patients with gastric precancerous lesions such as IM and gastric atrophy, in a low incidence area of GC, with high frequency of H. pylori infection. Patients with IM had higher levels of PG-II and lower PG-I/PG-II ratio. However, only 60% patients with IM had lower PG-I/PG-II ratio, suggesting that this test may not be useful for non-invasive diagnosis of IM in low-incidence area of GC, endemic for H. pylori infection, such as India.
GC is globally the second commonest malignancy, and carries a poor prognosis.
[21] Only 1% people infected with H. pylori develop GC and more than 80% of adult population in India is infected with this pathogen. If GC can’t be prevented, it needs to be detected early through screening programs to achieve better prognosis for these patients, as has been accomplished in Japan.
[22] In India, the disease is often detected at an advanced stage leading to poor prognosis.
[3,
23] Correa et al
[4] proposed a model for multi-step mechanism of gastric carcinogenesis following infection with H. pylori that includes step-wise development of chronic active gastritis, chronic atrophic gastritis, IM, dysplasia, carcinoma-in-situ and finally advanced GC. In a recent study, we found that IM was detected in 101/252 (40%) patients with gastric neoplasm as compared to only 2/101 (2%) in controls.
[24]
Human PG-I and II are proenzymes of pepsin, an endoproteinase constituent of gastric secretions.
[25] The level of PG-I is uniformly high in the sera of healthy subjects and patients with non-atrophic gastritis. On the other hand PG-I levels have been noted to be consistently low in patients of atrophic gastritis. PG-II serum levels on the other hand, remains high even in presence of gastric atrophy, thus reducing the normal ratio of PG-I/PG-II.
[26,
27] Unlike non-atrophic gastritis, atrophic gastritis is often associated with IM, which is a precursor of GC.
[28] Thus, it has been proposed that serum PG measurements may be helpful in non-invasive diagnosis of various gastric pathologies including IM in patients with dyspeptic symptoms and these serum markers have been used in Japan as noninvasive ‘‘serologic biopsy’’ for GC screening programs.
[29] There is only one study from India which has examined this issue in 30 patients each with GC and dyspepsia.
[30]
However, the study did not evaluate the utility of PG-I/PG-II ratio for non-invasive diagnosis of IM and their small sample size didn’t offer any definitive conclusions.
Gastrin stimulates parietal cells of the stomach to secrete hydrochloric acid (HCl). As stomach acidity rises, gastrin production normally decreases. But various gastric abnormalities such as atrophic gastritis, IM and GC are associated with unusually high levels of serum gastrin.
[31,
32] Also, H. pylori infected patients have been noted to have significantly higher gastrin levels.
[33,
34] However, in the present study G-17 was comparable among H. pylori positive and negative patients, suggesting that G-17 is unlikely to be a useful non-invasive screening test.
The need for validating a non-invasive marker of IM in Indian population can’t be over-emphasized. GC is often diagnosed at an advanced stage resulting in high mortality. H. pylori infection is common in Indian adults and the frequency of development of GC is lower in India in contrast to Japan.
[3,
24,
35]
Thus, subjecting all dyspeptic patients with H. pylori infection to upper gastrointestinal endoscopy is unlikely to be useful and cost-effective in India. Furthermore, available evidence suggests that H. pylori infection may not be the only independent factor responsible for gastric carcinogenesis. The host’s genetic make-up and dietary factors play a major role in determining whether a person infected with H. pylori will develop gastric atrophy, IM and/or gastric cancer? This has major implications for GC preventive strategies. Therefore, early non-invasive detection of IM as a precursor lesion of GC, which
develops due to several known and unknown host factors and host-agent interactions, is expected to be of major importance in early detection of GC and institution of preventive strategies in subjects with high-risk for development of this potentially fatal malignancy.
Our study however, showed that the PG-I/PG-II ratio is not as promising as has been reported from other areas of the world with lower frequency of H. pylori infection and higher frequency of GC.36 Such discordance in findings might be related to the fact that the predictive value of any test reduces with declining prevalence of the disease. Frequency of GC in general is low in India and northern India in particular. Furthermore, high frequency of H. pylori infection among our control group with dyspepsia might also result in lower PG-I/ PG-II ratio among them, thus affecting the discriminative ability of this test.
In conclusion, though the PG-I/PG-II ratio was lower in our patients with IM, only 60% of them had low ratio, suggesting that this test and G-17 levels may not be useful for early noninvasive detection of IM in low-incidence areas of GC, endemic for H. pylori infection.
Acknowledgements
UCG thanks the Indian Council of Medical Research, New Delhi for providing financial support for this study through project No. 5/4/3-1/2007-NCD-II; and SK thanks the University Grants Commission for awarding the fellowship F. No. 10-2(5)/2007(ii)- E.U.II, to the author.
References
- Singh K, Ghoshal UC. Causal role of Helicobacter pylori infectionin gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346–51.
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
- Ghoshal UC, Chaturvedi R, Correa P. The enigma of Helicobacter pylori infection and gastric cancer. Indian J Gastroenterol. 2010;29:95–100.
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40.
- Pasechnikov VD, Chukov SZ, Kotelevets SM, Mostovov AN, Polyakova MB, Mernova VP. The non-invasive diagnosis of precancerous changes of stomach mucosa. Rocz Akad Med Bialymst. 2004;49:66–71.
- Basson MD, Modlin IM. Pepsinogen: biological and pathophysiologic significance. J Surg Res. 1988;44:82–97.
- Samloff IM, Stemmermann GN, Heilbrun LK, Nomura A. Elevated serum pepsinogen I and II levels differ as risk factors for duodenal ulcer and gastric ulcer. Gastroenterology. 1986;90:570–6.
- Hassan MI, Toor A, Ahmad F. Progastriscin: structure, function, and its role in tumor progression. J Mol Cell Biol. 2010;2:118–27.
- Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971;61:185–8.
- Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973;65:36–42.
- Miki K, Sasajima M. Pepsinogen I and pepsinogen II, PG I/II ratio. Nippon Rinsho. 2011;68:778–81.
- Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103–31.
- Kikuchi R, Abe Y, Iijima K, Koike T, Ara N, Uno K, et al. Low serum levels of pepsinogen and gastrin 17 are predictive of extensive gastric atrophy with high-risk of early gastric cancer. Tohoku J Exp Med. 2011;223:35–44.
- Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer—recent mechanistic update. Mol Cell Biochem. 2003;253:329–38.
- Walker V, Taylor WH. Cigarette smoking, chronic peptic ulceration, and pepsin 1 secretion. Gut. 1979;20:971–6.
- Lauren P. The Two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification.Acta Pathol Microbiol Scand. 1965;64:31–49.
- Talley NJ, Lam SK, Goh KL, Fock KM. Management guidelines for uninvestigated and functional dyspepsia in the Asia-Pacific region: First Asian Pacific Working Party on Functional Dyspepsia. J Gastroenterol Hepatol. 1998;13:335–53.
- Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504–9.
- Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
- Ghoshal UC, Ghosh TK, Ghoshal U, Shujaatullah F, Banerjee K, Mazumder DN. In-house rapid urease test kit and commercial kit: which is better? Indian J Gastroenterol. 1999;18:183.
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6.
- Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–8.
- Ghoshal UC, Tripathi S, Ghoshal U. The Indian enigma of frequent H. pylori infection but infrequent gastric cancer: is the magic key in Indian diet, host’s genetic make up, or friendly bug? Am J Gastroenterol. 2007;102:2113–4.
- Ghoshal UC, Tiwari S, Dhingra S, Pandey R, Ghoshal U, Tripathi S, et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215–22.
- Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–55.
- Oishi Y, Kiyohara Y, Kubo M, Tanaka K, Tanizaki Y, Ninomiya T, et al. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163:629–37.
- Kwak MS, Kim N, Lee HS, Lee HE, Jung HC, Song IS. Predictive power of serum pepsinogen tests for the development of gastric cancer in comparison to the histologic risk index. Dig Dis Sci. 2010;55:2275–82.
- di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523–30.
- Leung WK,Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87.
- Parthasarathy G, Maroju NK, Kate V, Ananthakrishnan N, Sridhar MG. Serum pepsinogen I and II levels in various gastric disorders with special reference to their use as a screening test for carcinoma stomach. Trop Gastroenterol. 2007;28:166–70.
- Leja M, Kupcinskas L, Funka K, Sudraba A, Jonaitis L, Ivanauskas A, et al. The validity of a biomarker method for indirect detection of gastric mucosal atrophy versus standard histopathology. Dig Dis Sci. 2009;54:2377–84.
- Kang JM, Kim N, Yoo JY, Park YS, Lee DH, Kim HY, et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter. 2008;13:146–56.
- Chourasia D, Misra A, Pandey R, Ghoshal UC. Gastric atrophy and intestinal metaplasia in a patient on long-term proton pump inhibitor therapy. Trop Gastroenterol. 2008;29:172–4.
- Chourasia D, Ghoshal UC. Pathogenesis of gastro-oesophageal reflux disease: what role do Helicobacter pylori and host genetic factors play? Trop Gastroenterol. 2008;29:13–9.
- Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307–19.
- Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479–86.