|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
Portal hypertension, children, biliopathy, growth, sclerotherapy. |
|
|
Ujjal Poddar, Vibhor Borkar
Department of Pediatric Gastroenterology
Sanjay Gandhi Postgraduate Institute of Medical Sciences
Lucknow, India
Corresponding Author:
Dr. Ujjal Poddar
Email: ujjalpoddar@hotmail.com
DOI:
http://dx.doi.org/
Abstract
Extrahepatic portal venous obstruction (EHPVO) is the commonest cause of portal hypertension and variceal bleeding in children. Though mortality related to variceal bleeding is uncommon, morbidity due to massive splenomegaly with hypersplenism, growth failure, ectopic varices like rectal varices and portal biliopathy is significant. A significant proportion of cases in adults are due to procoagulant state but the same has not been documented in children. Studies in children have shown that hereditary or acquired coagulation disorders do not play a role in the pathogenesis of EHPVO in children. Regarding endotherapy for variceal bleeding, there is no doubt that band ligation is superior to sclerotherapy. Nevertheless, a combination of band ligation followed by sclerotherapy has shown to be superior to either modality in children with EHPVO. Growth retardation due to growth hormone resistance is common in children with EHPVO. Diminished portal blood flow results in decreased insulin delivery to the liver and thereby decreased production of insulin-like growth factor-1 (IGF-I) and insulin-like growth factor binding protein-3 (IGFBP-3). Improvement of growth after restoration of hepatic blood flow with mesenteric-left-portal bypass or Rex shunt, has been documented. Portal biliopathy is universal in adults and common in children but symptomatic cases are mainly in adults; thereby suggesting a progressive nature of the condition. Symptomatic biliary obstruction can be managed endoscopically but shunt surgery followed by biliary bypass (if necessary) seems to be the best management option. With the availability of the most physiological shunt (mesenteric-left-portal bypass or Rex) the management paradigm of EHPVO has changed from endotherapy to primary shunt surgery.
|
48uep6bbphidvals|415 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Extrahepatic portal venous obstruction (EHPVO) is the commonest cause of portal hypertension in children[1,2] and one of the common causes in adults in India.[3] In a study of 517 children from Chandigarh we have shown that EHPVO was responsible for 54% cases of portal hypertension (including bleeders and non-bleeders) and 85% of cases of portal hypertension with variceal bleeding.[1] Similarly, a study from Lucknow in 75 children showed that 95% cases of upper gastrointestinal bleeding was variceal and EHPVO was the cause in 92% of them.[4] The majority of children (85% to 90%) with this condition present with variceal bleeding, which they tolerate well.[1,4] While mortality due to variceal bleeding is uncommon, significant morbidity due to massive splenomegaly with hypersplenism, growth failure, ectopic varices like rectal varices and portal biliopathy is seen with EHPVO. Hence the management of EHPVO is no longer restricted to the management of variceal bleeding alone; it encompasses the management of growth failure, portal biliopathy, colorectal varices, massive splenomegaly with its associated complications like infarction, hypersplenism, physical disability due to pain, early satiety etc. In this article we will discuss the management of EHPVO under the headings of etiology, management of variceal bleeding, ectopic varices, portal biliopathy, growth failure and the role of shunt surgery.
Etiology of EHPVO/pro-coagulant state/ anticoagulation therapy in children
There are many postulated etiologies of EHPVO namely umbilical sepsis, umbilical vein catheterization, abdominal trauma, surgery, intra-abdominal sepsis, dehydration, congenital agenesis or atresia of portal vein. However, in India the majority of cases (90%) are idiopathic.[5] Recently, it has been documented that hypercoagulable states play an important role in the pathogenesis of EHPVO in a significant proportion of cases in adults and they are either due to deficiencies of natural anticoagulants like protein-C, protein- S, anti-thrombin III or excess production of procoagulants due to factor V Leiden or prothrombin gene mutations. Studies in children with EHPVO have shown that the deficiencies of antithrombotic factors (protein-C, protein-S, anti-thrombin III) are frequent but unlikely to be genetic in origin.[6,7] In a study in 19 children, Yachha et al[6] have shown that 42% had low protein-C levels and another 33% had high anti-cardiolipin antibody levels. In another study in 20 children with EHPVO, Dubuisson et al[7] have shown that almost half of the patients had protein- C, protein-S and anti-thrombin III deficiencies but their levels were normal in all parents, suggesting that these deficiencies are not genetic in origin. In a follow up study from our institute we have shown that factor V Leiden and prothrombin gene mutations were uncommon in children with EHPVO.[8] Out of 49 children with EHPVO none had prothrombin gene mutations and only 1 case and 2 of 49 healthy controls had factor V Leiden mutations. In adults, overt or occult primary myeloproliferative disorders (MPD) are the commonest cause of portal vein thrombosis. In an elegant study from King’s College it has recently been documented that JAK2 V617F mutation for MPD was not seen in any of the thirty pediatric cases with EHPVO.[9] Hence, hereditary or acquired coagulation disorders do not play an important role in the pathogenesis of EHPVO in children and the etiology is not known in most cases. Low hepatic blood flow due to portal vein thrombosis (low synthesis) and portosystemic shunt (increased clearance or consumption) are supposed to be responsible for low anticoagulant proteins in blood in EHPVO. The latter mechanism has been further substantiated by the fact that after conventional shunt surgery the levels of anticoagulant proteins tend to further go down.[7,10] The etiopathogenic role of low hepatic blood flow in depreciation of anticoagulant proteins has been substantiated by improvement in their levels after restoration of intrahepatic portal venous blood flow with a mesenterico-left portal vein bypass (MLPVB) or Rex shunt.[11] Of the 11 children who received Rex shunt, 10 had protein-C and protein-S deficiencies before surgery but after one year of surgery their levels normalized in all except one who had shunt stenosis. On the other hand, in two children who received conventional (distal splenorenal) shunt, the protein-C values became worse after shunt surgery (61% to 48% activity). Though the role of anticoagulation therapy remains controversial in adults,12 there is no role of anticoagulation therapy in children with EHPVO.
Management of esophageal varices
Almost 90% of children with EHPVO present with variceal bleeding and the remaining 10% with splenomegaly.[13,14] Unlike patients with chronic liver disease, those with EHPVO have a good prognosis as their liver functions are preserved. Mortality is mainly due to variceal bleeding and it is to the tune of 5%.[15] Therefore, treatment of EHPVO must be directed towards prevention of variceal bleeding and control of bleeding, should it occur.
There is no controversy about the management of acute variceal bleeding. After hemodynamic resuscitation all such patients should undergo endotherapy [either sclerotherapy (EST) or band ligation (EVL)]. However, controversy exists regarding prevention of further bleed. There are two main approaches to prevent further bleed: endoscopic intervention (EST, EVL) and shunt surgery. The issue of optimal treatment of children with EHPVO is not yet settled. Whether these patients should have shunt surgery after control of acute bleeding by endotherapy (EST or EVL) or should undergo further endotherapy for variceal eradication and surveillance is a long-standing debate. Conventional shunts (proximal or distal splenorenal) are not possible in almost one-third of cases due to blockage of splenic vein (SV) or small SV.[5,16] In this subset of patients endoscopic therapy is one of the few treatment options available. However, with the availability of new mesenterico-portal (mesenterico-left portal-bypass) or Rex shunt, the problems of conventional shunts have been largely overcome. As of now, we do not have sufficient data to say what proportions of EHPVO cases are suitable for this new shunt.
Endoscopic sclerotherapy (EST) or endoscopic band ligation (EVL)
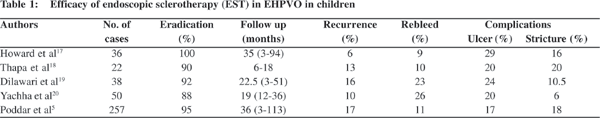
Of the two modalities of endotherapy, EST is an established modality to tackle varices in children (Table 1).5,17-20 The eradication rate of esophageal varices with EST is 88% to 100%. However, complications like ulcer (8% to 30%) and stricture (6% to 20%) are often encountered with EST. On the other hand, EVL has the advantages of rapid eradication of varices requiring fewer sessions and portending fewer complications. Indeed, EVL has become the preferred mode of treatment of variceal bleeding in adults. Unfortunately, the experience of EVL in children is limited.[21,22] The only randomized trial of EVL versus EST in children with EHPVO by Zargar et al,[22] showed that EVL was clearly superior to EST. Nevertheless, EST cannot be totally ruled out as a therapeutic modality, especially in children. EST remains the only therapeutic option in children younger than 2 years of age due to insertion difficulty with a comparatively larger banding cylinder. In many developing countries EST is still preferred over EVL due to the higher cost of the latter.
Endoscopic band ligation followed by sclerotherapy
Despite the clear cut benefit of EVL when used alone, there is a higher risk of recurrence of varices as it is difficult to ligate smaller varices, and because perforators and paraesophageal collaterals remain patent after EVL. Consequently recurrence of varices has been shown to be higher with EVL. In a study on EVL in children it has been shown that actuarial risk of variceal recurrence after 2 years was 40%.[21] To overcome this problem, the use of a combination of EVL followed by low dose EST has been suggested.[23] The idea is to take advantages of both EVL and EST. By using EVL as a primary treatment, one can achieve rapid eradication or down gradation of varices with fewer complications and by using low-dose EST following EVL, one can block perforators and paraesophageal collaterals, thereby reducing the risk of recurrence. In a study on 136 cases of EHPVO from Chandigarh (30 received EVL plus EST and 106 EST alone), we have shown that a combination of EVL with EST eradicated varices in all patients over fewer sessions (2 ± 1 vs. 4.4 ± 2, p<0.001) and there were significantly lesser complications in the combination therapy group (10% vs. 36%, p<0.01). Variceal recurrence was low in both the groups (6.6% in EVL plus EST group versus 10% in EST group over a follow up period of 27 months, p=ns) as both the groups were exposed to EST, thereby nullifying the effect of EVL alone.[24] In another study of 161 cases (EVL plus EST in 101, EST alone in 60) from Lucknow, we have shown similar results with EVL followed by EST versus EST alone.[25] It seems that EVL followed by EST is a better modality in children with EHPVO. However, further studies (especially comparison of EVL plus EST with EVL alone) are required to substantiate its superiority over EVL or EST alone.
Management of gastric varices and portal hypertensive gastropathy
Gastric varices: Earlier all attention was concentrated on esophageal varices in EHPVO because the main manifestation was variceal bleeding. With the availability of EST and EVL, esophageal varices can now be easily tackled, even in children. Hence, more attention is now being paid to the other effects of portal hypertension on the gastrointestinal tract, namely gastric varices and portal hypertensive gastropathy (PHG).26-30 However, most of the available literature is in adults and cirrhotic patients. There are only a few studies in children with EHPVO.[31,32,33] In a study of 274 cases of EHPVO from Chandigarh we have shown that 70% had gastric varices at presentation and 97% of them were gastroesophageal varices with only 3% isolated gastric varices.[32] This figure is higher than the reported figure in adults with EHPVO[26, 27] but similar to another study in children.[34] It has been shown that gastric varices bleed less frequently but when they do, they bleed profusely. The risk of bleeding is more with secondary gastric varices (varices appearing after eradication of esophageal varices), isolated gastric varices (IGV1) and gastroesophageal varices along the greater curvature of stomach (GOV2).26 In our study, we have shown that following eradication of esophageal varices, the prevalence of IGV1 increased significantly from 1% to 14% (p<0.001) and the incidence of bleeding from gastric varices increased from 0% to 20%.[32] Hence, the risk of development of high risk gastric varices (IGV1 and GOV2) increases significantly following obliteration of esophageal varices with concomitant increase in bleeding risk. This has been substantiated in other studies in children.[35, 36] Goncalves et al[35] showed that the incidence of gastric varices was the same in the sclerotherapy group and in controls, but bleeding from gastric varices occurred in the sclerotherapy group only. In a long term study of 36 children with EHPVO, Stringer et al[36] showed that following endoscopic obliteration of esophageal varices 12% patients required shunt surgery on follow up for bleeding gastric varices. Acute gastric variceal bleeding can be controlled with tissue glue (N-acetyl-2butyl- yanoacrylate) injection. However, in EHPVO unlike in cirrhosis where cyanoacrylate injection, beta-blockers or TIPS are used in econdary prophylaxis (preventing further bleed from gastric varices),37 large fundal varices are considered as an indication for shunt surgery as it provides a onetime treatment for the disease.
Portal hypertensive gastropathy (PHG): PHG classically described in cirrhosis. It may present with occult bleed or as an unusual cause of overt gastrointestinal bleeding. PHG has also been documented in EHPVO. In our series of 274 cases, 27% had mild PHG at presentation. However, following variceal eradication the prevalence of PHG as well as severe PHG increased significantly (PHG: 25% to 52%, p<0.001 and severe PHG: 3.2% to 16%, p<0.05) but no bleeding was noted from any case of PHG.[32] Similar results have been reported by Itha et al[34] in their study of 183 EHPVO cases. In adults, beta-blockers are recommended for patients who have bled from portal hypertensive gastropathy; and shunts (TIPS or surgical) are recommended in those patients in whom beta-blockers are contraindicated or have failed.[37] Fortunately a PHG bleed is uncommon in children with EHPVO. However, if there is bleeding from PHG shunt surgery should be considered as the benefit of long-term beta-blockers in EHPVO in children has not been proven in clinical trials.
Management of colorectal varices
The effect of increased portal pressure in EHPVO is not localized to the esophagus and stomach; it affects the entire gastrointestinal tract. Rectal varices are documented in 80 to 90% of adult EHPVO cases. Further the prevalence of rectal varices is more in EHPVO than in cirrhosis, probably due to the duration of portal hypertension or selective redistribution of portal pressure along the inferior mesenteric vein consequent to thrombosis at the junction of splenic and superior mesenteric veins.[38,39,40] There are suggestions that the severity of portal hypertension (large and/or bleeding esophageal varices) and sclerotherapy of esophageal varices may be associated with the development of rectal varices.38 Fortunately only a small proportion of patients with rectal varices (5%) present with rectal bleeding (Table 2).[38,39,40,41] Nevertheless, studies in children are scanty. Heaton et al[42] in a study on 14 children with EHPVO have documented rectal varices in 64% of cases and rectal varices were commoner with EHPVO than in intrahepatic causes of portal hypertension (26% of 46 cases). Four (7%) patients were noted to have bleeding from rectal varices and were managed with sclerotherapy and band ligation. In another study from Lucknow, Yachha et al[43] have documented rectal varices in 36% of 25 children by sigmoidoscopy and 76% by rectal endoscopic ultrasonography. The low prevalence in pediatric studies clearly suggests that the duration of portal hypertension plays an important role in the development of rectal varices. It is expected that the incidence of rectal varices with and without rectal bleeding will rise in children as they approach adolescence and adulthood. Bleeding from rectal varices can be managed with sclerotherapy or band ligation.[42] Shunt surgery[39] is considered in patients with large rectal varices with or without bleeding and with symptomatic colopathy.

Management of portal biliopathy
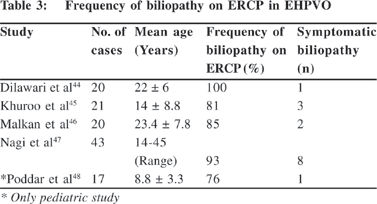
Portal biliopathy is the term used to describe cholangiographic abnormalities of the extrahepatic and intrahepatic bile ducts in patients with EHPVO. The prevalence of portal biliopathy in adults is almost 100% (Table 3)[44,45,46,47,48] but symptomatic biliary obstruction is present in just 5% to 19% of cases. In the only pediatric study so-far, reported by us in 17 cases from Chandigarh[48] we have documented biliary changes in 13 (76%) cases and only one child was symptomatic with biliary stricture. It has also been observed that while all symptomatic cases are adults but cholangiographic changes are equally evident in both children and adults,25 thereby implying that bile duct disease (portal biliopathy) in EHPVO may be progressive in nature and would manifest clinically in adulthood.[45]
The postulated mechanisms of biliary changes in EHPVO are extrinsic compression by portal collaterals,[44] ischemic stricture of bile duct due to injury at the time of portal venous thrombosis[45] or a combination of both.[49] There is evidence to support both theories of compression and ischemia. In a study of five cases, Dhiman et al[49] showed that repeat ERCP done after 4-8 weeks of shunt surgery demonstrated total disappearance of cholangiographic changes in one, partial disappearance in two and no change in remaining two cases. This indicates while in some patients compression plays an important role, in others ischemia alone or in combination with compression causes biliary changes. In another study Chaudhary et al[50] documented relief of jaundice in five of seven patients within 3-7 weeks of shunt surgery and the remaining two patients required second stage hepaticojejunostomy. This observation again supports the compression theory in most cases of portal biliopathy.
The definitive method of diagnosis of portal biliopathy is ERCP. However, since most of the patients are asymptomatic, this approach is recommended only if a therapeutic intervention is contemplated. Magnetic resonance (MR) imaging with intravenous gadolinium injection delineate the cavernoma and biliary changes simultaneously and may be extremely useful in children.[51] However, experience with MR in children is limited and more studies are required before recommending it for routine use.
Symptomatic portal biliopathy is a definite indication for intervention. Primary biliary tract surgery has significant morbidity and mortality due to extensive collaterals around the bile ducts. Some people believe that shunt surgery should be done first in all cases with symptomatic biliopathy and if it fails to resolve biliary obstruction then second stage biliary surgery is recommended.[50] Others believe that endoscopic management should be the first modality and if obstruction persists despite endotherapy, only then shunt surgery should be resorted to.[52] Patients with choledocholithiasis without associated biliary stricture can successfully undergo endoscopic sphincterotomy and stone extraction. Patients with choledocholithiasis and stricture will require multiple sessions of endoscopic therapy with balloon dilatation and stent placement. In patients with endoscopic failure, a staged procedure (portosystemic shunt followed by biliary surgery) should be preferred.[52] There are no clear guidelines for timing of biliary surgery following shunt. A longer interval (up to 1 year) and documentation of a patent shunt with decompressed collaterals on color Doppler or MR angiography may help in determining the optimum time for surgery.
The only pediatric study by Gauthier-Villars et al[53] in 8 children with symptomatic biliopathy in EHPVO showed regression of cholestasis in all cases after shunt surgery (mesocaval shunt in 6 and Rex in 2). Following surgery, serum aminotransferases and gamma glutamyl transpeptidase (GGT) activities returned to normal levels within 1 to 6 weeks in 5 children and remained normal on follow-up from 5 to 15 years. Liver function tests took 2 to 2.5 years to normalize in the remaining 3 cases. After a follow up of 4.5 to 15 years, all children were alive and displayed no dilatation of bile ducts on abdominal ultrasonography except one who had partial regression. Hence, at least in children shunt surgery is indicated in EHPVO with symptomatic portal biliopathy. Shunt surgery should be considered for asymptomatic portal biliopathy in presence of another indication like growth failure, symptomatic hypersplenism or ectopic varices.
Growth failure in EHPVO
Growth retardation is common in children with EHPVO. In a study of 61 children with EHPVO, Sarin et al[54] showed that 51% of children were short for their age (height< 90% of expected), compared with 16% of matched controls (p<0.01). Another study from Lucknow[55] documented growth retardation (height less than 5th percentile for age) in 54.5% of 33 children with EHPVO versus 5.7% of 35 controls (p<0.001). In the former study, it was found that despite comparable energy intake in cases and controls, growth velocity decreased in 73% children with EHPVO on follow up. This suggests that children with EHPVO have growth failure and decreased growth velocity despite adequate nutrition.[54] Mehrotra et al[55] found that children with EHPVO had significantly lower mid arm circumference but the triceps skinfold thickness was comparable to healthy controls. These findings suggest diminished anabolic action of growth hormone on muscle growth affecting the lean muscle mass, and its lipolytic effect resulting in decreased adiposity.
The exact mechanism of the growth failure is not known. Two possible mechanisms have been proposed. The first hypothesis contends growth failure to be a consequence of poor substrate utilization or/and malabsorption due to portal hypertensive enteropathy. Menon et al[56] reported that relief of portal hypertension by surgical porto-systemic shunts resulted in improved growth in 76% of 30 children with EHPVO, which supports the idea that portal enteropathy and subsequent malabsorption are in part the cause of growth impairment. On the other hand, the alternate hypothesis suggests that shunting of blood away from the liver results in impaired synthesis of factors needed for normal growth. Significantly increased levels of growth hormone and decreased levels of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) have been noted in EHPVO patients, suggesting growth hormone resistance.[55,57]
Diminished portal blood flow due to portal vein thrombosis results in decreased insulin delivery to the liver and thereby decreased production of IGF-I and IGFBP-3. Growth parameters have been shown to be improved significantly at 12 and 24 months follow up after restoration of blood flow to the liver and decrease in portal hypertension by mesenterico-left portal vein bypass (MLPVB) in children.[58,59] However, there are few studies showing improvement in growth hormone parameters following Rex shunt thus supporting growth hormone resistance as the underlying mechanism of growth failure in children with EHPVO. Growth retardation constitutes a relative indication for mesenterico-portal bypass (Rex shunt) surgery.
Shunt surgery in EHPVO
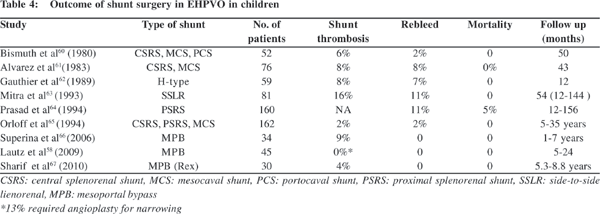
Conventionally, medical and endoscopic management is usually recommended for EHPVO, and various surgical shunts are used for refractory or complicated cases. Surgery is primarily indicated when endotherapy fails to control bleeding, in presence of gastric or ectopic varices not amenable to endoscopic management and with delayed sequelae like portal biliopathy and rectal varices. Emergency shunt surgeries have become a rarity in the era of endoscopic management. Other indications of shunt surgery include symptomatic hypersplenism, growth retardation, portal biliopathy, massive splenomegaly affecting the quality of life, rare blood group, and remote area of residence. Various studies have shown shunt patency in 85% to 98% patients (Table 4)[58,60,61,62,63,64,65,66,67] with long term survival in >95% patients with conventional portosystemic shunts (proximal splenorenal shunt, central splenorenal shunt, side-to-side lienorenal shunt and mesocaval shunt).
There is no report of post shunt encephalopathy in EHPVO cases and re-bleed occurs only when the shunt is blocked. The mesoportal bypass (MPB) is a new shunt that restores mesenteric blood flow to the liver through the Rex venous recessus (portion of left portal vein joining the umbilical vein). In this shunt, the patient’s own internal jugular vein is usually used as a conduit to bypass blood from superior mesenteric vein to left branch of portal vein. This is the most physiological shunt as it restores the hepatic blood flow. Besides correcting portal hypertension, it also abolishes the systemic manifestations of EHPVO such as normalization of coagulation parameters,11 improved liver function[66] and linear growth.[58] In fact with the help of Rex shunt, EHPVO can now be cured.
The debate is whether to send a child for shunt surgery (preferably Rex shunt) immediately after controlling acute variceal bleeding with endotherapy or to continue endotherapy to prevent further bleed and to offer shunt surgery as and when the child needs it. It seems there is a paradigm shift in EHPVO management towards shunt surgery after the introduction of Rex shunt as it provides complete cure of the condition.[68] In fact it has been suggested that MLPVB should be performed early after the diagnosis as the ability of the portal venous system to adapt to restored flow is agedependent as the deprivation of blood flow leads to atrophy of intrahepatic portal veins. Irreversible changes in the portal vein may decrease the compliance of the portal vein for adaptation if the shunt is done late.[66]
Long-term outcome of EHPVO
In general the prognosis of children with EHPVO is good. Longterm studies after endotherapy have shown almost no mortality. In a study of 36 children Stringer et al36 noted over a mean follow up of 8.7 years that 70% children remained asymptomatic but 30% had recurrence of bleeding. Half of the re-bleed cases were managed with endotherapy for esophageal varices while the remaining half had to be undertaken for shunt surgery for bleeding gastric varices. Zargar et al69 followed up 59 children after variceal eradication for 15 (10 to 20) years and documented recurrence of bleeding in 12% of cases (one third of them from gastric varices). Most of these bleeding episodes occurred within the first 4 years of variceal eradication. In another study, Thomas et al70 followed up 198 patients of EHPVO for 20 (14 to 23) years after eradication and showed re-bleeding in 17% of cases (25% from gastric varices) after a mean interval of 5.4 years but less than 10 years from eradication.
Conclusion
EHPVO is the commonest cause of portal hypertension and variceal bleeding in children. With the availability of effective endotherapy, the mortality due to variceal bleeding has become a rarity but the morbidity due to late sequelae like growth failure, portal bilipathy, gastric varices, rectal varices etc. is significant. There is a paradigm shift in EHPVO management towards shunt surgery after the introduction of mesenteric-left-portal vein bypass or Rex shunt as it provides complete cure of the condition.
References
- Poddar U, Thapa BR, Rao KL, Singh K. Etiological spectrum of esphageal varices due to portal hypertension in Indian children: is it different from the West? J Gastroenterol Hepatol.2008;23:1354–7.
- Poddar U, Thapa BR, Puri P, Girish CS, Vaiphei K, Vasishta RK, et al. Non-cirrhotic portal fibrosis in children. Indian J Gastroenterol. 2000;19:12–3.
- Dilawari JB, Chawla YK. Extrahepatic portal venous obstruction. Gut. 1988;29:554–5.
- Yachha SK, Khanduri A, Sharma BC, Kumar M. Gastrointestinal bleeding in children. J Gastroenterol Hepatol. 1996;11:903–7.
- Poddar U, Thapa BR, Singh K. Endoscopic sclerotherapy in children: experience with 257 cases of extrahepatic portal venous obstruction. Gastrointest Endosc. 2003;57:683–6.
- Yachha SK, Aggarwal R, Sharma BC, Misra RN, Aggarwal A, Naik SR. Functional protein C and anti–cardiolipin antibody in children with portal vein thrombosis. Indian J Gastroenterol. 2001;20:47–9.
- Dubuisson C, Boyer-Neumann C, Wolf M, Meyer D, Bernard O. Protein C, protein S and antithrombin III in children with portal vein obstruction. J Hepatol. 1997;27:132–5.
- Sharma S, Kumar SI, Poddar U, Yachha SK, Aggarwal R. Factor V Leiden and prothrombin gene G20210A mutations are uncommon in portal vein thrombosis in India. Indian J Gastroenterol. 2006;25:236–9.
- Abd El-hamid N, Taylor RM, Marinello D, Mufti GJ, Patel R, Mieli-Vergani G, et al. Aetiology and management of extrahepatic portal vein obstruction in children: King’s College Hospital experience. J Pediatr Gastroenterol Nutr. 2008;47:630–4.
- Fisher NC, Wilde JT, Roper J, Elias E. Deficiency of natural anticoagulant proteins C, S and antithrombin in portal vein thrombosis: a secondary phenomenon? Gut. 2000;46:534–9.
- Mack CL, Superina RA, Whitington PF. Surgical restoration of portal flow corrects procoagulant and anticoagulant deficiencies associated with extrahepatic portal vein thrombosis. J Pediatr. 2003;142:197–9.
- Chawla Y, Duseja A, Dhiman RK. Review article: the modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30:881–94.
- Arora NK, Lodha R, Gulati S, Gupta AK, Mathur P, Joshi MS, et al. Portal hypertension in north Indian children. Indian J Pediatr. 1998;65:585–91.
- Mittal SK, Kalra KK, Aggarwal V. Diagnostic upper GI endoscopy for hematemesis in children: experience from a pediatric gastroenterology centre in north India. Indian J Pediatr. 1994;61:651–4.
- Mowat AP. Prevention of variceal bleeding. J Pediatr Gastroenterol Nutr. 1986;5:679–87.
- Koshy A, Bhasin DK, Kapoor KK. Bleeding in extrahepatic portal vein obstruction. Indian J Gastroenterol. 1984;3:13–4.
- Howard ER, Stringer MD, Mowat AP. Assessment of injection sclerotherapy in the management of 152 children with oesophageal varices. Br J Surg. 1988;75:404–8.
- Thapa BR, Mehta S. Endoscopic sclerotherapy of esophageal varices in infants and children. J Pediatr Gastroenterol Nutr. 1990;10:430–4.
- Dilawari JB, Chawla YK, Ramesh GN, Mitra SK, Walia BN. Endoscopic sclerotherapy in children. J Gastroenterol Hepatol. 1989;4:155–60.
- Yachha SK, Sharma BC, Kumar M, Khanduri A. Endoscopic sclerotherapy for esophageal varices in children with extrahepatic portal vein obstruction; a follow -up study. J Pediatr Gastroenterol Nutr. 1997;24:49–52.
- McKiernan PJ, Beath SV, Davison SM. A prospective study of endoscopic esophageal variceal ligation using multiband ligator. J Pediatr Gastroenterol Nutr. 2002;34:207–11
- Zargar SA, Javid G, Khan BA, Yattoo GN, Shah AH, Gulzar GM, et al. Endoscopic ligation compared with sclerotherapy for bleeding esophageal varices in children with extrahepatic portal venous obstruction. Hepatology. 2002;36:666–72.
- Sarin SK, Gupta R. Endoscopic ligation plus sclerotherapy: two plus two makes only three! Gastrointest Endosc. 1999;50:129–33.
- Poddar U, Thapa BR, Singh K. Band ligation plus sclerotherapy versus sclerotherapy alone in children with extrahepatic portal vein obstruction. J Clin Gastroenterol. 2005;39:626–9.
- Poddar U, Bhatnagar S, Yachha SK. Endoscopic band ligation followed by sclerotherapy: Is it superior to sclerotherapy in children with extrahepatic portal venous obstruction? J Gastroenterol Hepatol. 2011;26:255–9.
- Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–9.
- De BK, Ghoshal UC, Das AS, Nandi S, Mazumdar DN. Portal hypertensive gastropathy and gastric varices before esophageal variceal sclerotherapy and after obliteration. Indian J Gastroenterol. 1998;17:10–2
- Sarin SK, Shahi HM, Jain M, Jain AK, Issar SK, Murthy NS. The natural history of portal hypertensive gastropathy: influence of variceal eradication. Am J Gastroenterol. 2000;95:2888–93.
- Soin AS, Acharya SK, Mathur M, Sahani P, Nundy S. Portal hypertensive gastropathy in non-cirrhotic patients. The effect of lienorenal shunts. J Clin Gastroenterol. 1998;26:64–7; discussion 68.
- Sarin SK, Misra SP, Singal A, Thorat V, Broor SL. Evaluation of the incidence and significance of the “mosaic pattern” in patients with cirrhosis, non-cirrhotic portal fibrosis and extrahepatic obstruction. Am J Gastroenterol. 1988;83:1235–9.
- Yachha SK, Ghoshal UC, Gupta R, Sharma BC, Ayyagari A. Portal hypertensive gastropathy in children with extrahepatic portal venous obstruction: role of variceal obliteration by endoscopic sclerotherapy and Helicobacter pylori infection. J Pediatr Gastroenterol Nutr. 1996;23:20–3.
- Poddar U, Thapa BR, Singh K. Frequency of gastropathy and gastric varices in children with extrahepatic portal venous obstruction treated with sclerotherapy. J Gastroenterol Hepatol. 2004;19:1253–6.
- Hyams JS, Treem WR. Portal hypertensive gastropathy in children. J Pediatr Gastroenterol Nutr. 1993;17:13–8.
- Itha S, Yachha SK. Endoscopic outcome beyond esophageal variceal eradication in children with extrahepatic portal venous obstruction. J Pediatr Gastroenterol Nutr . 2006;42:196–200.
- Goncalves ME, Cardoso SR, Maksoud JG. Prophylactic sclerotherapy in children with esophageal varices: long-term results of a controlled prospective randomized trial. J Pediatr Surg. 2000;35:401–5.
- Stringer MD, Howard ER. Long term outcome after injection sclerotherapy for esophageal varices in children with extra hepatic portal hypertension. Gut. 1994;35;257–9.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–76.
- Chawla Y, Dilawari JB. Anorectal varices—their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut. 1991;32:309–11.
- Ganguly S, Sarin SK, Bhatia V, Lahoti D. The prevalence and spectrum of colonic lesions in patients with cirrhosis and noncirrhotic portal hypertension. Hepatology. 1995;21:1226–31.
- Misra SP, Dwivedi M, Misra V, Dharmani S, Kunwar BK, Arora JS. Colonic changes in patients with cirrhosis and in patients with extrahepatic portal vein obstruction. Endoscopy. 2005;37:454–9.
- Goenka MK, Kochhar R, Nagi B, Mehta SK. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol. 1991;86:1185–9.
- Heaton ND, Davenport M, Howard ER. Incidence of haemorrhoids and anorectal varices in children with portal hypertension. Br J Surg. 1993;80:616–8.
- Yachha SK, Dhiman RK, Gupta R, Ghoshal UC. Endosonographic evaluation of the rectum in children with extrahepatic portal venous obstruction. J Pediatr Gastroenterol Nutr. 1996;23:438–41.
- Dilawari JB, Chawla YK. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–6.
- Khuroo MS, Yattoo GN, Zargar SA, Javid G, Dar MY, Khan BA, et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–13.
- Malkan GH, Bhatia SJ, Bashir K, Khemani R, Abraham P, Gandhi MS, et al. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–8.
- Nagi B, Kochhar R, Bhasin D, Singh K. Cholangiopathy in extrahepatic portal venous obstruction. Radiological appearances. Acta Radiol. 2000;41:612–5.
- Poddar U, Thapa BR, Bhasin DK, Prasad A, Nagi B, Singh K. Endoscopic retrograde cholangiopancreatography in the management of pancreaticobiliary disorders in children. J Gastroenterol Hepatol. 2001;16:927–31.
- Dhiman RK, Puri P, Chawla Y, Minz M, Bapuraj JR, Gupta S, et al. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–52.
- Chaudhary A, Dhar P, Sarin SK, Sachdev A, Agarwal AK, Vij JC, et al. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–9.
- Condat B, Vilgrain V, Asselah T, O’Toole D, Rufat P, Zappa M, et al. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–8.
- Khare R, Sikora SS, Srikanth G, Choudhuri G, Saraswat VA, Kumar A, et al. Extrahepatic portal venous obstruction and obstructive jaundice: approach to management. J Gastroenterol Hepatol. 2005;20:56–61.
- Gauthier-Villars M, Franchi S, Gauthier F, Fabre M, Pariente D, Bernard O. Cholestasis in children with portal vein obstruction. J Pediatr. 2005;146:568–73.
- Sarin SK, Bansal A, Sasan S, Nigam A. Portal-vein obstruction in children leads to growth retardation. Hepatology. 1992;15:229–33.
- Mehrotra RN, Bhatia V, Dabadghao P, Yachha SK. Extrahepatic portal vein obstruction in children: anthropometry, growth hormone and insulin-like growth factor I. J Pediatri Gastroenterol Nutr. 1997;25:520–3.
- Menon P, Rao KL, Bhattacharya A, Thapa BR, Chowdhary SK, Mahajan JK, et al. Extrahepatic portal hypertension: quality of life and somatic growth after surgery. Eur J Pediatr Surg. 2005;15:82–7.
- Nihal N, Bapat MR, Rathi P, Shah NS, Karvat A, Abraham P, et al. Relation of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels to growth retardation in extrahepatic portal vein obstruction. Hepatol Int. 2009;3:305–9.
- Lautz TB, Sundaram SS, Whitington PF, Keys L, Superina RA. Growth impairment in children with extrahepatic portal vein obstruction is improved by mesenterico-left portal vein bypass. J Pediatr Surg. 2009;44:2067–70.
- Stringer MD. Improved body mass index after mesenterico-portal bypass. Pediatr Surg Int. 2007;23:539–43.
- Bismuth H, Franco D, Alagille D. Portal diversion for portal hypertension in children. The first ninety patients. Ann Surg. 1980;192:18–24.
- Alvarez F, Bernard O, Brunell F, Hadchouel P, Odièvre M, Alagille D. Portal obstruction in children. II. Results of surgical portosystemic shunts. J Pediatr. 1983;103:703–7.
- Gauthier F, De Dreuzy O, Valayer J, Montupet P. H-type shunt with an autologous venous graft for treatment of portal hypertension in children. J Pediatr Surg. 1989;24:1041–3.
- Mitra SK, Rao KLN, Narasimhan KL, Dilawari JB, Batra YK, Chawla Y, et al. Side-to-side lienorenal shunt without splenectomy in noncirrhotic portal hypertension in children. J Pediatr Surg. 1993;28:398–401; discussion 401–2.
- Prasad AS, Gupta S, Kohli V, Pande GK, Sahni P, Nundy S. Proximal splenorenal shunts for extrahepatic portal venous obstruction in children. Ann Surg. 1994;219:193–6.
- Orloff MJ, Orloff MS, Rambotti M. Treatment of bleeding esophagogastric varices due to extrahepatic portal hypertension: results of portal-systemic shunts during 35 years. J Pediatr Surg. 1994;29:142–51; discussion 151–4.
- Superina R, Bambini DA, Lokar J, Rigsby C, Whittington PF. Correction of extrahepatic portal vein thrombosis by the meserteric to left portal vein bypass. Ann Surg. 2006;243:515–21.
- Sharif K, Mckiernan P, de Ville de Goyet J. Mesoportal bypass for extrahepatic portal vein obstruction in children: close to cure for most! J Pediatr Surg. 2010;45:272–6.
- Superina R, Shneider B, Emre S, Sarin S, de Ville de Goyet J. Surgical guidelines for the management of extra-hepatic portal vein obstruction. Pediatr Transplant. 2006;10:908–13.
- Zargar SA, Yattoo GN, Javid G, Khan BA, Shah AH, Shah NA, et al. Fifteen-year follow up of endoscopic injection sclerotherapy in children with extrahepatic portal venous obstruction. J Gastroenterol Hepatol. 2004;19:139–45.
- Thomas V, Jose T, Kumar S. Natural history of bleeding after esophageal variceal eradication in patients with extrahepatic portal venous obstruction; a 20-year follow-up. Indian J Gastroenterol. 2009;28:206–11.
|
|
|
 |
|
|