48uep6bbphidvals|424
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Post-transplant malignancies (PTMs) are emerging as a major challenge in patients successfully treated by organ transplant. Immunosuppressed allograft recipients have three to five fold increase in cancer risk as compared to age matched controls.[1] The three most common malignancies in allograft recipients are nonmelanotic skin cancers, Kaposi’s sarcoma and lymphoproliferative disorders.[2]
Post-transplant lymphoproliferative disorders (PTLD) are reported in up to 10% of solid organ transplant recipients.[3]
WHO has classified PTLD as: (i) Early lesion characterized by reactive plasmacytic hyperplasia or infectious mononucleosis type picture; (ii) Polymorphic PTLD showing full range of B cell maturation from immunoblast to plasma cell, small to medium lymphocyte and cells resembling centrocytes; and (iii) Monomorphic monoclonal PTLD, which includes B cell neoplasms like diffuse large B cell lymphoma, Burkitt’s Tropical Gastroenterology 2011;32(2):136–138lymphoma, plasma cell myeloma, T cell lymphoma and Hodgkin’s lymphoma.4 PTLD is reported in 1-3 % patients after renal and liver transplant, 2-6% in heart-lung transplants and 20% in small bowel transplants.[3] Majority of cases arise from B cells and T cell variants are rare.[3,5]
We report a rare case of late onset T cell lymphoma of periampullary region in a post-renal transplant patient.
Case Report
A 48 year old male, known case of hypertension and type II diabetes mellitus was admitted to the hospital with complaints of fever since one month, along with abdominal pain and occasional vomiting. He had undergone renal transplant in 1988, at the age of 27 years. The patient suffered from pulmonary tuberculosis in 2002, for which he was treated with complete anti-tubercular therapy. The patient was on azathioprine and steroids for the renal allograft.
On examination, he was pale and had icterus. No lymph nodes were palpable. On thorough investigation, his liver functions were deranged with serum bilirubin 5.2 mg%, SGOT 94 IU/ml, ALP 1385 IU/ml and GGT 2568 IU/ml. MRCP showed the intra-hepatic bile radicals to be dilated with a mass lesion in distal common bile duct with thickening of duodenal wall. This was confirmed on endoscopy which revealed wall of the duodenum to be thickened. Bone marrow examination showed mild plasmacytosis. Endoscopic biopsy was performed. It showed superficial ulceration of duodenal epithelium. The lamina propria was infiltrated by abundant lymphoid infiltrate.

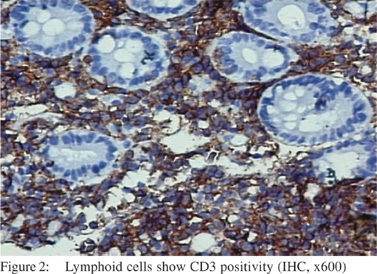
This infiltrate was composed of monomorphic cells. The individual cells were large sized with scanty rim of cytoplasm and moderately pleomorphic nuclei with dispersed chromatin and indistinct nucleoli (Figure 1). A few neutrophils were seeninterspersed. Few blood vessels with prominent endothelial cells were seen. On imunohistochemistry the infiltrate showed diffuse positivity with CD45, CD3 and CD30 (Figure 2 & 3), while CD20 and ALK-1 were negative. A diagnosis of posttransplant anaplastic large T cell lymphoma of peri-ampullary region was made.
The patient’s family was told about the prognosis and treatment plan, but while they could decide on their future course, the patient died within 15 days of the diagnosis.

Discussion
Post-transplant lymphoproliferative disorders are most common malignancies, apart from skin cancers, in solid organ transplant recipients.[3] Immunosuppressive drugs greatly increase the risk of post transplant malignancies by impairing cancer surveillance and facilitating the action of oncogenic agents, including viruses.[6] Most studies implicate that PTLD incidence went up after increasing usage of cyclosporine, while some other contradict this view.[7]Cyclosporin may cause cancer progression through direct cellular effect and also by transforming growth factor-a (TGF a).
In-vitro studies havesuggested that cyclosporine induces apoptosis and suppression of DNA repair. Newer immunosuppressive agents that are proliferative signal inhibitors, like sirolimus and everolimus, are being considered to have a role in prevention, modification and even treatment of PTMs.[8] Our patient was on azathioprine and steroids for renal allograft.
In our patient, PTLD was of monomorphic type and showed diffuse positivity for T cell marker (CD3). CD30 was also positive indicating this to be of anaplastic large cell lymphoma (ALCL) type. PTLDs are predominantly B cell type, accounting for about 80% of the cases. T cell lymphomas account for about 15 % of all types of PTLD; while ALCL is even rarer. T cell PTLDs tend to occur late in the post transplant period. Most of the cases present after five years, with less than 10% in the first year.[9] In comparison, B cell PTLDs tend to occur during first year of transplantation; when immunosuppression is highest. Our patient presented after 20 years transplantation, which is more than most of the earlier reported cases. Also, majority of T cell PTLDs present at extra-nodal sites like liver, spleen, skin, kidney, central nervous system and gastrointestinal tract.[9]
Role of EBV infection in B cell PTLD is well established. Immunosuppression in the post transplant period can lead to profound decrease in EBV specific T cell surveillance and latently EBV infected B cells escape immunosurveillance and proliferate.[10] Recipients who are EBV negative and receive grafts from EBV positive individuals are at higher risk of developing PTLDs.3 Raised IL-10 levels and a unique monoclonal protein have been identified in patients who developed PTLD.3 HTLV-1 virus infection has been implicated in the pathogenesis of T cell PTLD. A case of T cell PTLD has been reported from Japan, in which HTLV-1 genome was detected by PCR and southern blot. Our patient was found to be negative for EBV DNA.
Prognosis in post transplant T cell PTLD remains poor. Majority of earlier cases have survived for less than 6 months.9 Non-responsive cases and aggressive lymphomas are treated by chemotherapy. Our patient expired within 15 days of the diagnosis thus verifying the poor prognosis.
References
- Dantal J, Pohanka E. Malignancies in renal transplantation: an unmet medical need. Nephrol Dial Transplant. 2007;22:i4–10.
- Zafar SY, Howell DN, Gockerman JP. Malignancy after solid organ transplantation: an overview. Oncologist.
2008;13:769–78.
- LaCasce AS. Post-transplant lymphoproliferative disorders. Oncologist. 2006;11:674–80.
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplastic diseases of hematopoietic and lymphoid tissue: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1999. Histopathology. 2000;36:69–86.
- LeBlond V, Sutton L, Darent R, Davi F, Bitker MO, Gabarre J, et al. Lymphoproliferative disorders after organ transplantation: a report of 24 cases observed in a single center. J Clin Oncol. 1995;13:961–8.
- Tanner JE, Alfieri C. The Epstein-Barr Virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV and the immune system in disease pathogenesis. Transpl Infect Dis. 2001;3:60–9.
- Birkeland SA, Hamilton-Dutoit S. Is post-transplant lymphoproliferative disorder (PTLD) caused by any specific
immunosuppressive drugs or by the transplantation per se? Transplantation. 2003;76:984–8.
- Pascual J. Post- transplant lymphoproliferative disorder— the potential of proliferation signal inhibitors. Nephrol Dial Transplant. 2007;22 suppl 1:i27–35.
- Rajakariar R, Bhattacharyya M, Norton A, Sheaff M, Cavenagh J, Raftery MJ, et al. Post transplant T- cell lymphoma : a case series of four patients from a single unit and review of literature. Am J Transplant. 2004;4:1534–8.
- Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Haematol. 2005;56:155–67.