48uep6bbphidvals|390
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Serotype variation due to environmental biological displacements in Vibrio cholerae El Tor diarrhea in northern Nigeria[1] was reported from 1975 to 1986 when laboratory surveillance was undertaken. In 1976 and 1978, epidemics due to Hikojima serotype occurred, disappearing in the following years, except in 1981. The Hikojima epidemic of 1978 was documented with endotoxaemia associated with clinical features.[2] Interest in determining the role of sialic acid as a marker in serum or as index of severity began during the 1978 epidemic.
Sialic acid is a carbohydrate moiety found on the outermost end of the glycan chains of cells and molecules in most animals including humans.[3] As a result of its unique location on the surface of cells and its chemical characteristics, sialic acid has been suggested to be vital in mediating cellular and molecular interactions as well as in the protection of cells and molecules from damage and premature apoptosis.[3,4] Sialic acid is also considered necessary in the regulation of immune response as it may serve as a basis for differentiating self from non-self antigens.[5] It has also been shown to play a role in the pathogenesis of many infectious and non-infectious diseases including cancers and cardiovascular diseases such as ischaemic heart disease.[4,6,7]
Sialisidase, also called neuraminidase, is an enzyme that functions to cleave sialic acid from the surface of cells and molecules.[3] Free sialic acids are either transferred onto a nascent glycan chain by an enzyme named siayltransferase or catabolized by acylneuraminate lyase into acylmannosamin and pyruvate.[3]
Neuraminidase is produced by a wide variety of pathogens including influenza virus, Trypanosoma spp, Clostridium perfrigens, Pseudomonas spp. and Vibro cholera.[4,6,8] By cleaving sialic acid on the surfaces of cells such as mucosal surfaces and blood cells, these pathogens may scavenge host sialic acid for nutrition. Furthermore, they may expose suitable microbial receptor sites on mucosal surfaces (e.g. influenza) or may disrupt the integrity of blood cells thereby facilitating clearance of affected blood cells from the circulation (e.g. Trypanosomiasis). Neuraminidase is also an important virulence factor exposing receptors for multiplication, spread and tissue invasion by pathogenic microorganisms (e.g. Clostridium perfrigens).[3]
Neuraminidase is produced by all toxigenic strains of Vibro cholera and it cleaves sialic acid from higher order ganglionsides on the intestinal mucosal surfaces unmasking the monosialosyl ganglionside (GMI ganglionside), which is the receptor for the cholera enterotoxin.[9] By increasing the expression of GMI receptor for cholera enterotoxin, neuraminidase enhances binding and internalization of the cholera toxin to increase severity of the secretory response to cholera enterotoxin.[9] Although cholera, mediated by enterotoxin, is primarily an acute intestinal disease characterized by watery rice-like stools, systemic complications such as anaemia, thrombocytopaenia and bacteraemia may occur.[2,9,10,11]
However, invasion is usually seen more often with non-01, non-0139 Vibro cholerae strains. Studies of cholera neuraminidase have revealed its relatively high binding affinity for surface sialic acids and its efficient lytic function[12] giving it the potential to influence the invasiveness of of cholera. This study, comparing serum sialic acid levels (an index of neuraminidase activity) in cholera cases and normal healthy controls was undertaken to determine if cholera neuraminidase crosses significantly into the circulation to cause in vivo cleavage of sialic acid.
Methods
During 1978 epidemic in Zaria, 20 consecutive patients with severe cholera (12 females, 8 males; mean age 32 years; range 22-40 years) were recruited before rehydration therapy was commenced at Ahmadu Bello University Teaching Hospital, Zaria. These patients were part of the reported epidemic that occurred in 1978[2]. The role of sialic acid was to be measured in subsequent epidemics due to Hikojima serotype. However, the rarity of Hikojima serotype infection in the subsequent years has prompted the current report of the data of 1978 epidemic. Twenty healthy adults (9 males, 11 females; mean age 33 years, range 25-42 years) hailing from same villages as the patients, were also recruited to serve as controls. Twenty milliliters (20 ml) of stool samples were collected from initial stool at the time of admission for stool pH and stool occult blood assessment. Five ml of blood samples were collected from all patients and controls; two ml of anti-coagulated blood were used for full blood count, differential while cell counts and platelet count as previously described.[2] Three ml of whole blood was spun at 2795g (RCF) and sera were frozen at -20oC until analysed for free sialic acid. Stool samples collected from 3 patients were frozen at -20oC until analysed for free sialic and neuraminidase activity.
Bacteriological diagnosis
Rectal swabs were taken from each patient, immediately inserted into universal bottles containing alkaline peptone, pH 8.6 and then cultured in thiosulphate citrate bile salt sucrose (TCBS) medium (Difco, USA). Acid production was indicated by a yellow colour with bromothymol blue indicator. Difco rabbit Vibrio cholerae 01 (Detroit, Michigan, USA) antisera were used for indentification. The isolates agglutinated chicken red blood cells. The Vibro cholerae causing epidemic was identified as El Tor biotype, Hikojima serotype.
Assay of free serum and stool sialic acid
Serum and stool sialic acid levels were determined by the thiobarbituric acid method.[13]
Assay of neuraminidase activity in stool
Neuraminidase activity in 3 stool samples was assayed with the method of Webster and Campbell,[14] in which the amount of sialic acid liberated from the substrate fetuin was determined. Briefly, 500 mg of freeze dried fetuin, type III (Sigma Chemical Company, St. Louise USA) was dissolved in 20 ml of 0.2M sodium phosphate citrate buffer, pH 6.7. The fetuin was prepared fresh; 0.5 ml of fetuin solution was added to 0.5 ml of cholera stool sample and 0.5 ml of phosphate citrate buffer was added to another 0.5 ml of cholera stool to serve as control. Samples were run in duplicate, incubated at 37oC in a water bath for 30 minutes. The amount of sialic acid liberated was compared to a blank and to standard N-acetyl-neuraminic acid type VI from Escherichia coli (Sigma Company, USA). Consent were obtained from all study participants and from the ethical committee of ABUTH, Zaria. Statistical analysis was carried out using SPSS 11. Quantitative variables were compared using student t-test and p<0.05 was considered significant.
Results
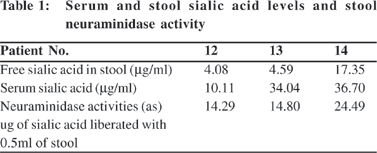
Free serum sialic acid in the patients ranged from 7.2 – 36.7 mg per ml (mean 15.04 + 9.31) and in controls from 4.26 – 31.38 mg per ml (mean 13.9 + 6.67),. The difference between the means was not statistically significant (p>0.05). Table 1 shows corresponding sialic acid levels in sera and stool of three patients and neuraminidase activity (in mg of sialic acid liberated) in the stool of the same patients.
The pH of all the stool samples ranged from 7 to 9. Stool occult blood assessed by the Haemacombistix reagent strip was present in small quantity (+) in 12 patients, moderate quantity (++) in 5 patients and large quantity (+++) in 3 patients. The mean haemoglobin of patients and controls were 11.7 + 1.65 g/dl (range 8-12) and 12.4 + 2.1 g/dl (range 10-14) respectively(p>0.05). The mean platelet count of patients and controls were 164.9 + 70.4 x 109/l (range 97-275) and 257.2 + 71.6 x 109/l (range 152-410) respectively, (p=0.002). Six (30%) of the 20 patients had a platelet count below 100 x 109/l. The mean total white cell count of patients were 112.0 + 5.16 x 109/l (range 6.4 – 24.5) with 81-84% neutrophilia, 8-15% lymphocytes and 0.3% monocytes, while those of controls were 6.18 + 2.54 x 109/ l (range 3.8-11.4) with 40-78% neutrophils, 20-52% lymphocytes and 1-2% monocytes. The mean total WBC count was significantly higher in patients than controls (p=0.001).
Discussion
The results show that free serum sialic acid levels in cholera patients and normal controls do no differ significantly and that cholera neuraminidase is liberated in the gut of Nigerian patients as found in the stool samples. This finding may suggest that neuraminidase liberated from Vibrio micro-organism in the gut may be mostly engaged against the glycoproteins in the bowel mucosa,[15] and an insignificant quantity, if any, enters the general circulation. However, the enzyme sialytransferase present in serum and cell membrane may rapidly transfer sialic acid onto nascent glycan chains of cells[3] or free sialic acid acids might have been catabolised by acylneuraminidase lyase,[3] if any cleavage does occur.
Temporal relationships between neuraminidase activity and free serum sialic acid levels have been shown in animal studies. In experimental Trypanosoma vivax infection in cattle, the neuraminidase-induced increase in serum sialic acid coincided with decrease in erythrocytic surface sialic acid on day 8 of infection.[8] Similarly, Savannah brown bucks experimentally infected with Trypanosoma evansi, were detected with serum neuraminidase activity which increased gradually, achieving significant levels on day 7, while free serum sialic acid levels became significantly higher in infected bucks compared to controls after 15 days.[16] Since cholera in man is a short lived clinical illness (usually less than 36 hrs), serial measurements of sialic acid could not be done in our study to establish whether serial rising of sialic acid ever occurred, especially as rapid rehydration therapy of patients had to be instituted.
The finding of peripheral blood leucocytosis and red blood cells in the stool of these Nigerian cholera patients is in concordance with other studies[17,18] and challenges the classical view that cholera diarrhea is non-inflammatory.[19] Many recent studies have also revealed that there is both systemic and mucosal inflammation in cholera as elevated levels of inflammatory mediators such as myeloperoxidase, lactoferrin, leucotrienes, tumour necrosis factor a and nitric oxide have been demonstrated in stool, blood and mucosal biopsies of cholera patients.[20,21] Furthermore, experimental animal studies using cholera toxin have also suggested that various interleukins, especially of the T helper 2 (Th2) cytokine arm, are elicited in the blood during cholera infection.[22,23]
It may be suggested that local vasculopathy, perhaps due to locally acting cholera endotoxins (lipopolysaccharide/LPS),[8] as well as inflammation-induced local sequestration of blood cells contributed to this finding of red blood cells in stool samples and perhaps the decrease of red blood cells and platelet counts in the circulation of some of our patients. Indeed, duodenal mucosa of cholera patients, in other studies, show extensive microvascular changes including endothelial damage, intravascular thrombosis, aggregation of red cells and platelets, as well as capillary obstruction by leucocytes.[24]
In conclusion, the role of cholera neuraminidase seems established at the local gut level where it cleaves sialic acid to expose GMI ganglioside receptors for the cholera toxin. This study indicates that neuraminidase may not reach the circulation in significant levels to contribute to significant cleavage of sialic acid in circulating blood cells of Nigeria patients infected with with Vibrio cholera El Tor Hikojima
serotype.
References
1. Onyemelukwe GC, Lawande RV. Serotype variation in Vibrio cholerae El Tor diarrhoea in northern Nigeria. Cent Afr J Med. 1991;37:186–9.
2. Onyemelukwe GC, Onuora C, Lawande RV, Onyewotu II, Mba EC, Mohammed I. Endotoxaemia in Vibro El Tor cholera. Trans R Soc Trop Med Hyg. 1982;76:590–4.
3. Traving C, Schauer R. Structure, function and metabolism of sialic acids. Cell Mol Life Sci. 1998;54:1330–49.
4. Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–60.
5. Bagriacik EU, Miller KS. Cell surface sialic acid and the regulation of immune cell interactions: the neuraminidase effect reconsidered. Glycobiology. 1999;9:267–75.
6. Pilatte Y, Bignon J, Lambre CR. Sialic acids as important molelcules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993;3:201–18.
7. Soong G, Muir A, Gomez MI, Waks J, Reddy B, Planet P, et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest. 2006;116:2297–305.
8. Esievo KA, Saror DI, Ilemobade AA, Hallaway MH. Variation in erythrocyte surface and free serum sialic acid concentrations during experimental Trypanosoma vivax infection in cattle. Res Vet Sci. 1982;32:1–5.
9. Galen JE, Ketley JM, Fasano A, Richardson SH, Wasserman SS, Kaper JB. Role of Vibro cholerae neuraminidase in the function of cholera toxin. Infect Immun. 1992;60:406–15.
10. Issa H, Shorman M, Bseiso B, Al-Salem AH. A case of 01 Vibro cholerae bacteraemia and primary peritonitis in a patient with liver cirrhosis. Gastro Res. 2009;2:358–60.
11. Restrepo D, Huprikar SS, VanHorn K, Bottone EJ. 01 and non- 01 Vibro cholerae bacteraemia produced by hemolytic strains. Diagn Microbiol Infect Dis. 2006;54:145–8.
12. Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, et al. Sialic acid recognition by Vibro cholerae neuraminidase. J Biol Chem. 2004;279:40819–26.
13. Aminoff D. Methods for the quantitative estimation of Nacetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961;81:384–92.
14. Webster RG, Campbell CH. An inhibition test for identifying the neuraminidase antigen of influenza viruses. Avian Dis. 1972;16:1057–66.
15. Gascoyne N, van Heyningen WE. Unmasking of actual and potential receptor sites for cholera toxin in intestinal mucosa homogenates. J Infect Dis. 1979;139:235–6.
16. Shehu SA, Ibrahim NDG, Esievo KAN, Mohammed G. Role of erythrocytic surface sialic acid in inducing anemia in Savannah Brown bucks experimentally infected with Trypanosoma evansi. Vet Ashir. 2006;76:521–30.
17. Onyemelukwe GC, Kulkarni AG. Polymorphonuclear leucocytosis in patients with cholera. Cent Afr J Med. 1986;32:224–5.
18. Saha DR, Niyogi SK, Nair GB, Manna B, Bhattachanya SK. Detection of faecal leucocytes and erythrocytes from stools of cholera patients suggesting an evidence of an inflammatory response in cholera. Indian J Med Res. 2000;112:5–8.
19. Handa S. Cholera. eMedicine [serial online]. 2010 Dec [cited 2011 Feb]. Available from: http://emedicine.medscape.com/article/ 214911-overview
20. Qadri F, Raqib R, Ahmed F, Rahman T, Wenneras C, Das SK, et al. Increased levels of inflammatory mediators in children and adults infected with Vibro cholerae 01 and 0139. Clin Diagn Lab Immunol. 2002;9:221–9.
21. Qadri F, Bhuiyan TR, Dutta KK, Raqib R, Alam MS, Alam NH et al. Acute dehydrating disease caused by Vibro cholerae serogroup 01 and 0139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut. 2004;53:62–9.
22. Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL- 1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–14.
23. Lavelle E, Jarnicki A, McNeela E, Armstrong ME, Higgins SC, Leavy O, et al. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol. 2004;75:756–63.
24. Koshi R, Chandy G, Mathan M, Mathan VI. Vascular changes in duodenal mucosa in shigellosis and cholera. Clin Anat. 2003;16:317–27.