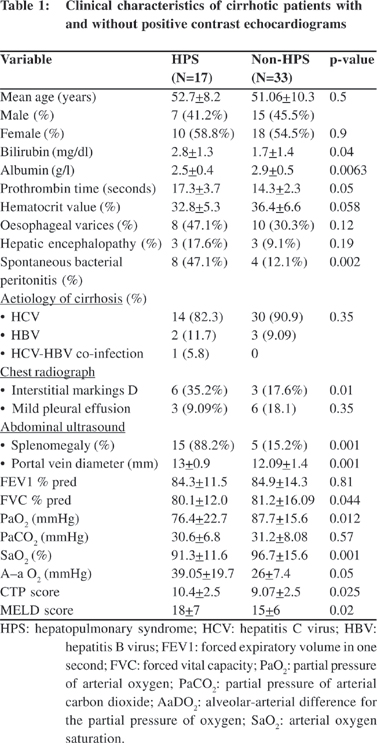
48uep6bbphidvals|384
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Pulmonary dysfunction including the hepatopulmonary syndrome (HPS) is an important complication of cirrhosis and portal hypertension which affects the treatment and disease prognosis.[1] Although HPS most commonly presents in patients with cirrhosis, it has also been reported among cases of chronic hepatitis with no cirrhosis or portal hypertension.[2]
Hepatopulmonary syndrome is defined as the triad of liver disease, pulmonary gas exchange abnormalities leading to arterial deoxygenation, and widespread intrapulmonary vascular dilatation (IPVD).[1,3] When cirrhotic patients have no sign of any cardiovascular disease, severe hypoxemia (PO2 < 60 mmHg) strongly indicates HPS.[4,5,6] A diagnosis of HPS is established when patients present with liver disease associated with IPVD and arterial gas exchange abnormalities (arterial– alveolar oxygen gradients A–a) O2) e” 15 mmHg or PaO2 < 80 mmHg).[3]
The prevalence of HPS in cirrhotic patients is very heterogeneous and varies across different geographic areas worldwide.[7,8,9,10,11] Despite the recent advances in our knowledge of HPS, and the high prevalence of liver cirrhosis in Egypt, mainly caused as a post viral hepatitis complication,[12,13] and due to Schistosomiasis induced portal hypertension,[14] little data is available about HPS in Egyptian patients. We therefore aimed to assess, (i) the prevalence of HPS in consecutive cirrhotic patients, (ii) the degree of pulmonary dysfunction in relation to liver function and (iii) the clinical and laboratory characteristics and their predictive values in diagnosis of this syndrome.
Methods
Patients
This study enrolled 82 consecutive cirrhotic patients referred to the Unit of Gastroenterology and Hepatology, Internal Medicine, Minia University Hospital, between December 2008 and February 2010. All patients participating in this study, were confirmed for liver cirrhosis by biopsy or clinical and laboratory evaluation. Transthoracic contrast echocardiography, arterial blood gas analysis on room air, lung function tests, and chest radiograph were done for all patients. Those with known cardiovascular and/or respiratory diseases and current history of smoking were excluded from this study. No patient had evidence of pulmonary hypertension when assessed by echocardiography,[15] including Doppler measurements.[16,17] The patients with ascites underwent large volume paracentesis before their enrollment in the study.
Thirty two patients were excluded from the study; nine patients because of inadequate echocardiographic image quality, ten patients due to non-feasibility of lung function tests, eight patients owing to current history of smoking and five patients because of impaired lung function tests, defined as forced expiratory volume in one second (FEV1) or total lung capacity (TLC) <66% of predicted.[18] The study protocol was approved by the Institutional Ethics Committee of School of Medicine, Minia University, Egypt, and all patients gave informed consent to participate in this study. The study was conducted in accordance with the ethical guidelines of the 1975 declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice.
Clinical and laboratory assessment
Physical examination was performed to detect clinical features including clubbing in fingers and toes, central and peripheral cyanosis, presence of spider angioma, telangiectasia, jaundice, collateral veins on abdominal wall, ascites, grade of encephalopathy, splenomegaly, dyspnea, peripheral edema, palmar erythema, and pleural effusion. All patients were tested for hepatitis B, hepatitis C, biliary, autoimmune, metabolic, cardiac, and alcoholic etiologies of chronic liver disease (CLD). Complete blood count (CBC), liver function test (LFT), creatinine, prothrombin time (PT), partial thromboplastin time (PTT), alpha-fetoprotein, albumin and other routine tests were undertaken in all patients. Ascitic fluid was tested for protein, albumin and white blood cells.
Procedure
All patients underwent a contrast enhanced transthoracic echocardiogram (CE),[19] using the Phillips HDI 1500® (Philips Medical Systems) equipped with 2.5 and 3.5 mHz transducers. Apical four-chamber imaging was used for the simultaneous visualization of the atria and ventricles. Peripheral venous access was obtained through the forearm of each patient using an i.v. catheter (20 g, 2.5 cm), with 0.9% saline solution. The procedure was performed by injecting agitated saline into patient’s right hand cubital vein. Left and right sides of the heart were evaluated by echocardiography after 5 beats. Presence of opacity after 5 beats in the left heart was determined as intrapulmonary shunt. If opacity was present immediately after injection, it was a sign of intracardiac shunt.[3] Three exams were performed on each patient and each subsequent injection was initiated after the disappearance of the microbubbles from all cavities. The results were recorded on videotape and analyzed by two examiners.
Arterial blood gas samples were obtained by percutaneous radial artery puncture with the subject in a seated position breathing room air, and were analysed with a standard blood gas analyser (BG Electrolytes, Instrumentation Lab. Inc., USA). Determination of A–a O2 was calculated as the difference between alveolar oxygen pressure (PAO2) and PaO2, in which PAO2 = [0.21 × (barometric pressure-47)] (1.25×PaCO2)].[20] In all patients, a second sample was taken after one hour in upright position, orthodeoxia was considered when a decrease in SaO2 of >4% after change from supine to the upright position (DSaO2).[21]
Patients were submitted to spirometry, using 2130 Spirometer (Vmax, Sensormedicus, USA) for the determination of forced vital capacity (FVC), forced expired volume in the first second (FEV1) and FEV1/FVC ratio.
Personnel performing and interpreting each of the studies were blinded to the results of the others.
Criteria for diagnosis of HPS
The patients presenting the three diagnostic criteria of hepatopulmonary syndrome, including hepatic cirrhosis, arterial blood deoxygenation (A–a O2 > 15 mmHg) and intrapulmonary arterial dilation were defined as clinical hepatopulmonary cases. Those presenting with intrapulmonary arterial dilation but no other two criteria (arterial blood hypoxemia) were defined as subclinical hepatopulmonary cases.[3]
Statistical analysis
The Student’s t-test for independent samples was employed for independent comparative analysis of the quantitative variables. For the qualitative variables, either Pearson’s chisquare test or Fisher’s exact test was used when appropriate. Sensitivity, specificity, positive and negative predictive values of clinical and paraclinical features in diagnosis of HPS were evaluated. All conclusions were made based on a significance level of 0.05.
Results
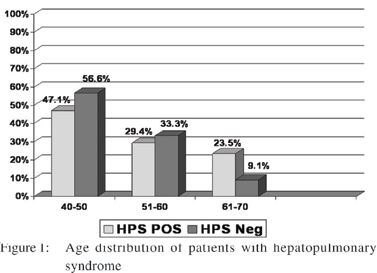
Among the 50 patients who participated in the study (mean age was 50 years (range 45–65); 22 (44%) were males and 28 (56%) were females. Etiology of CLD was hepatitis C virus (HCV) infection in 44 (88%) patients, hepatitis B infection (HBV) in 5 (10%) patients and HCV-HBV co-infection in one patient (2%) (Table 1). The presence of intrapulmonary vascular dilation (excluding other criteria of HPS) which was defined as subclinical hepatopulmonary syndrome and clinical HPS was observed in 10 (20%) and 17 (34%) patients, respectively. Most frequent age group was 41-50 years (Figure 1).
Baseline data of the subjects with and without HPS are shown in Table 1. Mean total bilirubin was significantly higher, and mean values for serum albumin, prothrombin time, and hematocrit value were significantly lower in cirrhotic patients with HPS. Complications of liver cirrhosis (oesophageal varices, hepatic encephalopathy and spontaneous bacterial peritonitis(SBP) were found more often in the group with HPS but only for SBP the difference approached statistical significance (p=0.002) (Table 1). Patients with HPS had more severe cirrhosis, assessed either by the Child-Pugh score (CP) or the Model for End-Stage Liver Disease (MELD) score (Tables 1 & 2).
Lung function values (FVC %, FEV1) were significantly lower (p=0.04) in cirrhotics with HPS when compared with those with those without HPS (p=0.81). The parameters of arterial oxygenation, PaO2 and A–a O2, revealed significantly high difference, with lower values in the group with HPS. PaCO2 showed no significant difference between the two groups. Chest radiograph showed interstitial markings, predominately in the lower lung fields, in six (35.2%) and three (9.09%) patients with and without HPS, respectively (p<0.01). Small pleural effusions were seen in three (17.6%) HPS patients and in six (18.1%) patients with and without HPS (p=0.35). In abdominal ultrasound, both portal vein diameter and splenomegaly were significantly wider (p=0.001) and more frequent (p=0.001) in cirrhotics with HPS compared with those without HPS.
Predictive factors for HPS
Assessment of frequency of clinical features in HPS patients, revealed dyspnea (82.4%), clubbing (64.7%) and cyanosis (58.8%) were the most prevalent. Dyspnea, cyanosis and clubbing were also the most sensitive and specific clinical features. Table 3 presents the characteristics and diagnostic values of signs and symptoms in HPS patients. No significant relation was found between ascites, edema, jaundice, oliguria, collateral veins and HPS (data not showed).
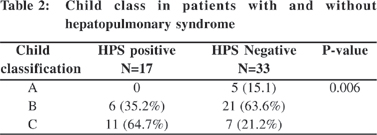

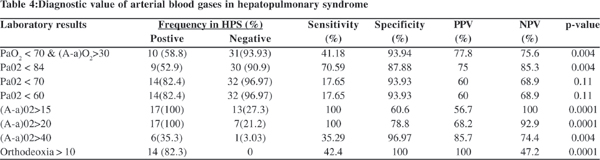
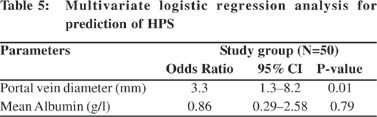
Table 4 presents the diagnostic value of arterial blood gas in HPS. The mean (orthodeoxia) DSaO2 in the HPS patients was 4.1 ± 1.8 compared to non-HPS patients, who showed no change (p=0.001). PaO2 and A–a O2 were most sensitive in diagnosis of HPS. Orthodeoxia specificity was 100%. By multiple logistic regression; portal vein diameter (mm) (OR 3.3; 95% C.I 1.3-8.2; p=0.01) was the only independent factor for prediction of HPS (Table 5).
Discussion
This prospective study demonstrated that 34 % of patients with CLD in Egypt had HPS based on established diagnostic criteria, and the prevalence of intrapulmonary shunting by echocardiography was 20%. Together these findings support that alterations in the pulmonary microvasculature are common
in cirrhosis.
In the literature, reports of occurrences of HPS in patients with cirrhosis has rates ranging from 4% to 32%.[7,8,9,10,11] The frequency among non-cirrhotic patients with portal hypertension has been found to be lower, ranging from 8% to 9.7%.[22,23] Among cases of chronic hepatitis with no cirrhosis or portal hypertension, the occurrence rate is 1.1%.[2] These considerable variations in frequency found in studies on HPS can be explained by different geographical origins of the study; the previous lack of standardization in the diagnostic criteria for defining HPS, due to the type of contrast used in the echocardiograms producing different diameters in the micro bubbles, and due to different thresholds used for the definition of de-oxygenation. The first suggestion for the standardization of diagnosis of HPS was in a paper published in 2004.[3] Our study clearly showed a significant correlation between the severity of HPS and Child-Pugh score and MELD score, suggesting that this syndrome is related with development of cirrhosis. Although, previous reports failed to find these association,[18,24] many recent reports confirmed the association of HPS and severity of liver disease.[5,9,25]
Although all complications of liver cirrhosis were found to be higher in patients with HPS rather than those without HPS; only SBP approached statistical significance (p=0.002). As, the etiopathogenesis of HPS remains unknown, these finding seem to be of interest. Intestinal endotoxemia accompanying cirrhosis may be an important factor with a double role in the development of both HPS[26] and SBP[27] providing a link between both phenomenon. Further prospective studies are needed to clarify the effect of SBP treatment on the incidence of HPS in patients with liver cirrhosis.
In our study HPS and severity of gas exchange were significantly related to portal hypertension. These findings which are similar to the data and conclusions arrived at by several recent studies,[6,11,23] support the hypothesis that HPS results from a disequilibrium between pulmonary vasoconstrictor and vasodilator substances particularly nitric oxide (NO). Intestinal endotoxemia may contribute in the pathogenesis of HPS by inducing NO. From pathophysiological point of view, HPS, SBP and portal hypertension seem to be closely interlinked.
Similar to previous reports, our study has also shown a relation between HPS and cyanosis, clubbing and dyspnoea. There was a significant correlation between cutaneous spider naevi and the severity of HPS. Spider naevi are significantly related to intrapulmonary venous dilation and gas exchangeabnormalities begetting further intrapulmonary vasodilatation.[28,29] Cyanosis, clubbing and dyspnoea had positive predictive value of 55.5%, 76%, and 66.6%, respectively in HPS, and negative predictive value of 91.3%, 93.9%, and 89.6%, respectively.
Although PaO2 has a good predictive value, A–a O2 is the most sensitive index for determining gas exchange abnormalities and A–a O2 >15mmHg at sea level (breathing ambient air) can be considered abnormal. Mean A–a O2 (mm Hg) and portal vein diameter (mm) were the only the independent factors for prediction of HPS [(OR, 1.1; 95% CI (1.01-1.2); p=0.031) and (OR, 3.3; 95% CI (1.3-8.2); p=0.01), respectively].
In summary, 34% of our patients hospitalized due to complications of CLD had HPS. Although these results were based on subset analysis and with the limitation of smaller sample size, these results suggest that HPS in Egypt is not less common, but is frequently under diagnosed, due to the fact that most of the affected patients are either asymptomatic or present vague complaints of dyspnoea and fatigue. Cyanosis and finger clubbing were found to be clinical markers for the presence of HPS. Even though not very specific, spider naevi were found to be a sensitive clinical indicator for the presence of IPVD. PaO2 and A–a O2 were most sensitive in diagnosis of HPS. There is a relationship of HPS with the severity of cirrhosis by Child’s grading and MELD score.
Acknowledgements
The authors thank Dr. Lamia Hamdy, Department of Clinical Pathology, Medical school, Minia University, Egypt, for her help in performing the laboratory analysis.
References
1. Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. Clin Chest Med. 2005;26:587–97, vi.
2. Teuber G, Teupe C, Dietrich CF, Caspary WF, Buhl R, Zeuzem S. Pulmonary dysfunction in non-cirrhotic patients with chronic viral hepatitis. Eur J Intern Med. 2002;13:311–8.
3. Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-Hepatic vascular disorders (PHD). Eur Respir J. 2004;24:861–80.
4. Lima BL, França AV, Pazin-Filho A, Araújo WM, Martinez JA, Maciel BC, et al. Frequency, clinical characteristics, and respiratory Hepatopulmonary syndrome 29 parameters of hepatopulmonary syndrome. Mayo Clin Proc. 2004;79:42–8.
5. Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, et al. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853–9.
6. Hira HS, Kumar J, Tyagi SK, Jain SK. A study of hepatopulmonary syndrome among patients of cirrhosis of liver and portal hypertension. Indian J Chest Dis Allied Sci. 2003;45:165–71.
7. Stoller JK, Lange PA, Westveer MK, Carey WD, Vogt D, Henderson JM. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation. The Cleveland Clinic experience. West J Med. 1995;163:133–8.
8. Shafiq M, Khan AA, Alam A, Butt AK, Shafqat F, Malik K, et al. Frequency of hepatopulmonary syndrome in cirrhotic patients. J Coll Physicians Surg Pak. 2008;18:278–81.
9. Ferreira PP, Camara EJ, Paula RL, Zollinger CC, Cavalcanti AR, Bittencourt PL. Prevalence of hepatopulmonary syndrome in patients with decompensated chronic liver disease and its impact on short-term survival. Arq Gastroenterol. 2008;45:34–7.
10. Zamirian M, Aslani A, Shahrzad S. Left atrial volume: a novel predictor of hepatopulmonary syndrome. Am J Gastroenterol. 2007;102:1392–6.
11. Møller S, Krag A, Madsen JL, Henriksen JH, Bendtsen F. Pulmonary dysfunction and hepatopulmonary syndrome in cirrhosis and portal hypertension. Liver Int. 2009;29:1528–37.
12. Egyptian Ministry of Health Annual Report: 2007. Egyptian Ministry of Health. [cited 2010 Mar 6]. Available from: http:// www.mohp.gov.eg
13. Khattab MA, Eslam M, Sharwae MA, Hamdy L. Seroprevalence of hepatitis C and B among blood donors in Egypt: Minya Governorate, 2000-2008. Am J Infect Control. 2010;38:640–1.
14. El-Zayadi AR. Curse of schistosomiasis on Egyptian liver. World J Gastroenterol. 2004;10:1079–81.
15. Schenk P, Globits S, Koller J, Brunner C, Artemiou O, Klepetko W, et al. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant 2000;19:145–54.
16. Kim WR, Krowka MJ, Plevak DJ, Lee J, Rettke SR, Frantz RP, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453–8.
17. Torregrosa M, Genesca J, Gonzalez A, Evangelista A, Mora A, Margarit C, et al. Role of Doppler echocardiography in the assessment of portopulmonary hypertension in liver transplantation candidates. Transplantation. 2001;:71:572–4.
18. Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305–10.
19. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
20. West JB. Pulmonary pathophysiology - the essentials. 4th ed. Baltimore: Williams and Wilkins; 1990.
21. Whyte MK, Hughes JM, Peters AM, Ussov W, Patel S, Burroughs AK. Analysis of intrapulmonary right to left shunt in the hepatopulmonary syndrome. J Hepatol. 1998;29:85–93.
22. Kaymakoglu S, Kahraman T, Kudat H, Demir K, Cakaloglu Y, Adalet I, et al. Hepatopulmonary syndrome in noncirrhotic portal hypertensive patients. Dig Dis Sci. 2003;48:556–60.
23. De BK, Sen S, Sanyal R. Hepatopulmonary syndrome in noncirrhotic portal hypertension. Ann Intern Med. 2000;132:924.
24. Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615–24.
25. Alizadeh AH, Fatemi SR, Mirzaee V, Khoshbaten M, Talebipour B, Sharifian A, et al. Clinical features of hepatopulmonary syndrome in cirrhotic patients. World J Gastroenterol. 2006;12:1954–6.
26. Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol. 2005;11:567–72.
27. Francés R, Muñoz C, Zapater P, Uceda F, Gascón I, Pascual S, et al. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860–4.
28. Robin ED, Horn B, Goris ML, Theodore J, Kessel AV, Mazoub J, et al. Detection, quantitation and pathophysiology of lung “spiders”. Trans Assoc Am Physicians. 1975;88:202–16.
29. Rodriguez-Roisin R, Roca J, Agusti AG, Mastai R, Wagner PD, Bosch J. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987;135:1085–92.