48uep6bbphidvals|388
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Biliary atresia (BA) is an idiopathic progressive inflammation and fibrosclerosis of the extra and intrahepatic bile ducts leading to biliary obstruction and jaundice.[1]
Despite successful surgery, progressive inflammation and fibrosis of intrahepatic bile ducts develops to varying degrees in all patients, leading to biliary cirrhosis in a vast majority of patients. Consequently, 70-80% of BA patients will eventually require liver transplantation, approximately half in the first 2 years of life.[2] It should be emphasized that it is the intrahepatic biliary lesion that determines overall prognosis and outcome, thus, this disease is no longer called extrahepatic BA. Without a better understanding of the etiology and pathogenesis of this intrahepatic sclerosing cholangitic process in BA, little progress can be expected in improving the non-transplant outcome of patients.[3]
It is now apparent that BA is a phenotype resulting from several pathogenic processes that culminate in obstruction of the biliary tree. The majority (80%) of cases of BA in western countries are of the perinatal or acquired form.[4] Although the etiology of this form is not completely understood, proposed precipitating factors include infectious, toxic, vascular, and immune mediators.[5]
The role of the immune system in bile duct injury and obstruction is poorly understood at present and is the focus of intense investigation. Activated effector T cells produce cytokines that can directly damage epithelial cells or indirectly damage them through stimulation of other immune cells. Investigation has revealed that BA is associated with Th1-cell-mediated portal tract inflammation. The periductular immune cells in BA produce IL-12, IFN-g, IL-2, and TNF-a.[6]
Inflammatory cytokines, such as IL-1b, IL-6 and tumor necrosis factor (TNF), play a pivotal role in the induction and maintenance of the systemic and local inflammatory response. They induce endothelial cells to express adhesion molecules and to produce chemokines, and are therefore responsible for the recruitment of inflammatory cells.[7] They are also responsible for the stimulation of both leukocytes and stromal cells (i.e. fibroblasts), leading to the production of tissue-damaging substances, such as metalloproteinases and oxygen radicals. In this respect, IL-8, whose production is induced by TNF-a and IL-6, is not only a potent recruiter of neutrophils and T cells but also a potent stimulator of the degranulation. Several studies have addressed the role of these cytokines in a variety of liver disease.[8]
The objective of this study was to measure the serum level of IL-6 and IL-8 as inflammatory cytokines in patients with BA and to evaluate their relation to the clinical outcome.
Methods
This study included 60 children attending the paediatric hepatology clinic and outpatient clinic, Children’s Hospital, Ain Shams University. They were classified into 3 groups; Group 1 included twenty children with biliary atresia. Group 2 included twenty children with intrahepatic cholestasis and Group 3 included twenty normal age and sex matched children as controls. This study was performed according to the recommendations of the Ethics Committee of Children’s Hospital, Ain Shams University. An informed consent was obtained from the children’s guardians.
All the included children were C-reactive protein (CRP) negative and patients with symptoms suggestive of any infectious disease were excluded from the study. All the biliary atresia patients received postoperative steroids 2 mg/kg /day for maximum duration of one month, antibiotic prophylaxis for cholangitis for one year, and all the cholestatic children were on ursodeoxycholic acid and fat soluble vitamins. A detailed history and clinical examination was done for all patients including parameters like: age, sex, and number of attacks of cholangitis after the surgery (for children with BA), presence or absence of jaundice, assessment of weight, size and consistency of the liver, size of the spleen below costal margin and the presence of ascites. The following laboratory investigations were done: CBC with differential count, liver function tests (total and direct bilirubin, INR, and albumin), and liver enzymes (ALT, AST, GGT). Abdominal ultrasonography was done for all patients. Liver biopsies performed at the time of diagnosis were re-evaluated for hepatic architecture, degree of fibrosis, and inflammatory cellular infiltrate. The gap between the liver biopsy and the measure of serum IL6 and IL8 ranged from 9 to 12 months. A repeat liver biopsy at the time of cytokine measurement was not done for ethical reasons. Serum IL-6 and IL-8 were measured by ELISA technique (AviBion, Oregnum Laboratories, Helsinki, Finland) in patients and controls. Five ml venous blood sample was collected from each subject, left to clot then centrifuged at 3000 rpm for 10 minutes and serum was separated. A part of serum was used for immediate analysis of liver function tests and CRP, while the remaining part was stored at – 70°C for subsequent assay of IL-6 and IL-8.
Statistical methods
Date was expressed as mean + SD for quantitative measures and both number and percentage for categorized data. Comparison between two independent mean groups for parametric data was done using student t test. For comparison between two independent groups for non-parametric data, Wilcoxon Rank Sum test (z) was used. Chi-square test was used to study the association between 2 variables or comparison between 2 independent groups for categorized data. Z-test for applied for comparison between 2 independent groups for categorized data. Analysis of Variance (ANOVA) and Multiple Comparison test were used for comparison between 3 groups. SPSS statistical software package was used for data analysis.
Results
Group 1 included 20 patients with biliary atresia; comprising of 11 females (55%) and 9 males (45%). Their ages ranged from 12 to 16 months with a mean age of 14.5 + 4.3 months. They were all operated by Kasai operation and their ages at the time of the operation ranged from 1.5 to 4 months with a mean age of 2.7 + 0.7 months. The number of attacks of cholangitis they had as a postoperative complication ranged from 1 to 4 times with a mean of 2 + 0.94 over one year of follow up. Group 2 included 20 patients with intrahepatic cholestasis, comprising of 8 females (40%) and 12 males (60%). Their ages ranged from 10 to 17 months with a mean of 12.1+4.6 months. The causes of intrahepatic cholestasis were progressive familial intrahepatic cholestasis (n=4), Alagille syndrome (n=3), non syndromic paucity of intrahepatic ducts (n=4) and neonatal hepatitis (n=9).
A cohort of 20 normal healthy children served as a control group, and included 8 females (40%) and 12 males (60%). Their ages ranged from 13 to 24 months with a mean of 17.8 + 4.8 months.
Serum AST, GGT and total bilirubin levels were significantly higher in group 1 compared to group 2 (156.9 + 78.3 vs. 110.3 + 48.8 IU/L, 206.3 + 198.9 vs. 104.8 + 133.9 IU/L, 12.9 + 3.1 vs. 10.5 + 2.6 mg/dl, respectively) while no significant differences could be found regarding the other parameters (Table 1).
Serum levels of IL-6 and IL-8 were significantly higher in patients with biliary atresia (394.7 + 40.2 and111.2 + 9.7 pg/dl respectively) when compared to patients with intrahepatic cholestasis (175.5 + 33.1and 53.5 + 8.2 pg/dl, respectively). The levels of both IL-6 and IL-8 were significantly higher in the 2 groups when compared to controls (30.4 + 8.8 and 13.7 + 4.1 pg/dl, respectively) (Table 2).
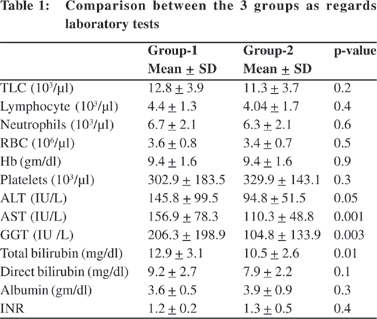
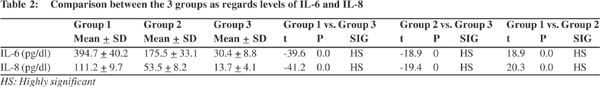
No significant correlation was found between levels of IL- 6 and any of the studied blood elements or the biochemical parameters in neither patients with biliary atresia nor patients with intrahepatic cholestasis. However a significant positive correlation between levels of IL-8 and lymphocytic count (r=0.5, p=0.04) and a significant negative correlation between levels of IL -8 and serum albumin (r=-0.5, p=0.04) could be detected in the biliary atresia group (Figures 1 & 2).
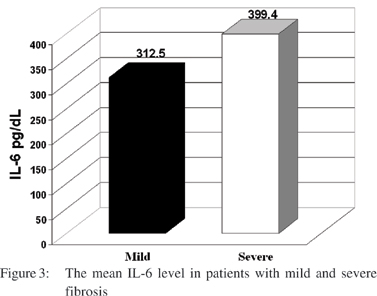
Significantly higher levels of IL-6 were found in children with biliary atresia having severe fibrosis as detected by liver biopsy than those with mild to moderate fibrosis (399.4 pg/dl vs. 312.5 pg/dl, respectively t=-2.2, p=0.04) (Figure 3); however no significant relation could be found between the degree of liver fibrosis or inflammatory cellular infiltrate in the liver biopsy neither with IL-6 nor IL-8 in those with intrahepatic cholestasis.
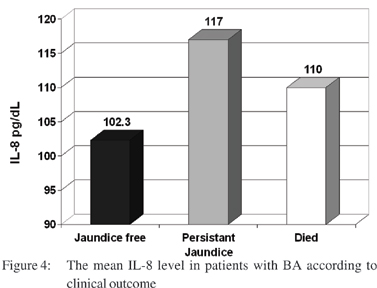
After one year of follow up, analysis of the outcome showed that 6 (30%) children with biliary atresia became free of jaundice, 10 (50%) had persistent jaundice and 4 (20%) patients died. The outcome of patients with intrahepatic cholestasis revealed that 7 (47%) patients became free of jaundice, 8 (53%) had persistent jaundice and no deaths among this group. There was no significant relation between levels of IL-6 and the outcome in children with biliary atresia or intrahepatic cholestasis. However there were highly significant higher levels of IL-8 in those with persistent jaundice than patients free from jaundice in BA group (p=0.01) (Figure 4).
Discussion
Biliary atresia is a rare disease of infancy, which has changed over the past 30 years from being fatal to being a disorder for which either effective palliative surgery or curative liver transplantation are available.[9] Little is known about the etiopathogenesis of biliary atresia. An interaction of genetic susceptibility and undefined perinatal exposure to infectious or toxic agents has been proposed.[10] Previous studies have demonstrated the expression of CD14 which is a cell surface glycoprotein for endotoxin recognition on activated Kupffer cells and liver sinusoidal endothelial cells in patients with biliary atresia. CD14 is a crucial inflammatory regulator in the liver that determines innate immunity to viral and bacterial pathogens and mediates production of TNF-a, IL-8, IL-6 and IL-12.[11]
The total bilirubin, AST and GGT levels were significantly higher in our children with biliary atresia when compared to those with intrahepatic cholestasis. Fishler et al[12] studied 30 patients with biliary atresia and 22 patients with intrahepatic cholestasis, and reported that total bilirubin, and GGT levels were higher in biliary atresia patients but they found no significant difference between the two groups regarding the AST levels. Higher AST levels in our study could be explained by the older age of our patients in comparison to those of Fishler et al as AST levels are related to the chronicity of the disease as stated by Visser et al.[13]
Higher levels of serum IL-8 (112.7 + 9.7 pg/dL) were found in our patients with biliary atresia when compared to controls (13.7 + 4.1 pg/dl) and those with intrahepatic cholestasis (53.5 + 8.2 pg/dl). Higher levels of serum IL-6 (394.7 + 40.2 pg/dL) were also found in our biliary atresia patients in comparison to controls (30.4+8.8 pg/dl) and intrahepatic cholestasis (175.5 + 33.1 pg/dl). IL-6 is secreted by T cells and macrophages. It favors mononuclear cell accumulation at the site of injury,through continuous monocyte chemoattractant protein (MCP)- 1 secretion. The increase in serum levels of IL-6 provides the basis for amplification step of chronic inflammation. IL-6 can activate endothelial cells to secrete IL-8, MCP-1 and induce expression of adhesion molecules.[14] IL-8 is secreted by macrophages and monocytes, and functions as the initial stimulus for recruitment of neutrophilis and T cells in the portal space. The release of lytic enzymes by activated neutrophilis and other proinflammatory cytokines by T lymphocytes may lead to inflammation in portal space and eventually to portal fibrosis.[11]
Honsawek et al[15] studied 60 patients with biliary atresia and found that serum levels of IL- 8 were markedly elevated in patients compared to controls and they suggest that measurement of IL-8 levels may possibly serve as a parameter for determining disease activity and may be predictive of the prognosis with respect to progression of liver dysfunction and portal hypertension in BA. Kobayashi et al[16] who studied serum levels of IL-6 in patients with biliary atresia at different ages after Kasai operation found levels of IL-6 to be high in patients with severe liver dysfunction than patients with stable liver function in all age groups. They concluded that serum levels of IL-6 may be predictive of prognosis with respect to the progression of fibrosis in postoperative biliary atresia. However Nobili et al[17] found that in patients with biliary atresia, levels of IL-6 were undetectable in the majority of samples with no significant differences from healthy controls while levels of IL-8 in patients with neonatal hepatitis were only marginally increased and were significantly lower than those found in biliary atresia. This suggested that increased IL-8 levels are not a generalized feature of liver disease in infancy and therefore it is conceivable to speculate that increased IL-8 production is a characteristic feature of biliary involvement. Interestingly Huang et al[18] studied levels of IL-6 and IL-8 in fresh liver tissues obtained from patients at the early and late stages of biliary atresia, and compared them with normal liver tissues serving as controls. Real–time quantitative reverse transcription polymerase chain reaction was used to quantitate IL-6 and IL-8 expression. There was significantly higher IL-8 expression in both the early and late stages of biliary atresia than in controls. There was also significantly higher expression of IL-6 expression in the late stage of biliary atresia than in controls but there was no significant difference between the early and late stages of biliary atresia as regards expression of IL-6 and IL-8. Moreover Chuang et al[19] stated that liver IL-8 mRNA increases in the late stage of BA, which is consistent with the studies that revealed increased serum IL-8 in BA, particularly, in the late stage in patients with persistent jaundice, portal hypertension or an increased histological activity index in the liver.[16,17]
There was no correlation between serum levels of IL-6 and any of the laboratory data (ALT, GGT, total and direct bilirubin) in our patients with intrahepatic cholestasis. Similarly Ding et al[20] reported that serum levels of IL-6 had no correlation with serum levels of (ALT, GGT, total and direct bilirubin) in their patients with intrahepatic cholestasis.
A significant positive correlation was evident between the serum IL-8 levels and lymphocytic count in our children with BA. Baggiolini et al[8] reported that both in rats with experimental obstructive jaundice and in humans with obstructive jaundice, circulating neutrophils showed hyperactivity. This may be a consequence of the elevated levels of IL-8 released in the peripheral circulation. Since IL-8 is also a chemo attractant for T lymphocytes, production of IL-8 may lead to the recruitment not only of neutrophils but also of T lymphocytes.
Serum levels of IL-6 in patients with severe fibrosis were significantly higher than patients with mild fibrosis among patients of biliary atresia, but there was no significant difference between patients with mild and severe fibrosis in the same group as regards levels of IL-8. On the other hand Honsawek et al[15] and Nobili et al[17] found that higher serum levels of IL-8 significantly correlated with the degree of fibrosis in patients of biliary atresia. They concluded that the increased production of IL-8 may be a mechanism leading to fibrosis in patients with chronic liver disease.
There was highly significant difference between patients with persistent jaundice and anicteric patients as regards levels of IL-8 in group of biliary atresia. This is similar to the study of Honsawek et al[15] who concluded that measurement of serum IL-8 levels may possibly serve as a parameter for determining
disease severity.
Narayanaswamy et al21 studied the role of proinflammatory cytokines in disease progression among patients with biliary atresia. They concluded that the circulating inflammatory process in biliary atresia is persistent, progressive and involves T cell, macrophages and cell adhesion molecules.
It is concluded from the present study that serum IL-6 and IL8 are high in patients with BA and may possibly indicate progressive inflammation. Measuring IL-6 and IL-8 levels y may help to determine disease severity and predict the progression to liver fibrosis in children with biliary atresia.
References
1. Kelly DA, Davenport M. Current management of biliary atresia. Arch Dis Child. 2007;92:1132–60.
2. Karrer FM, Bensard DD. Neonatal cholestasis. Semin Pediatr Surg. 2000;9:166–9.
3. Sokol RJ, Mack C. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R.
4. Perlmutter DH, Shepherd RW. Extrahepatic biliary atresia: a disease or a phenotype? Hepatology. 2002;35:1297–304.
5. Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21.
6. Mack CL, Tucker RM, Sokol RJ, Karrer FM, Kotzin BL, Whitington PF, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87.
7. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9.
8. Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv Immunol. 1994;55:97–179.
9. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet.2009;374:1704–13.
10. Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. SeminLiver Dis. 2001;21:517–24.
11. Bezzerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–9.
12. Fischler B, Papadogiannakis N, Nemeth A. Aetiological factors in neonatal cholestasis. Acta Paediatr. 2001;90:88–92.
13. Visser BC, Suh I, Hirose S, Rosenthal P, Lee H, Roberts JP, et al. The influence of portoenterostomy on transplantation for biliary atresia. Liver Transpl. 2004;10:1279–86.
14. Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25.
15. Honsawek S, Chongsrisawat V, Vejchapipat P, Thawornsuk N, Tangkijvanich P, Poovorawan Y. Serum interleukin-8 in children with biliary atresia; relation with disease stage and biochemical parameters. Pediatr Surg Int. 2005;21:73–7.
16. Kobayashi H, Yamataka A, Lane GJ, Miyano T. Levels of circulating antiinflammatory cytokine, interleukin-1 receptor antagonist and proinflammatory cytokines at different stages of biliary atresia. J Pediatr Surg. 2002;37:1038–41.
17. Nobili V, Marcellini M, Giovannelli L, Girolami E, Muratori F, Giannone G, et al. Association of serum interleukin-8 levels with the degree of fibrosis in infants with chronic liver disease. J Pediatr Gastroenterol Nutr. 2004;39:540–4.
18. Huang YH, Chou MH, Du YY, Huang CC, Wu CL, Chen CL, et al. Expression of toll-like receptors and type 1 interferon specific protein MxA in biliary atresia. Lab Invest. 2007;87,66–74.
19. Chuang JH, Chou MH, Wu CL, Du YY. Implication of innate immunity in the pathogenesis of biliary atresia. Chang Gung Med J. 2006;29:240–50.
20. Ding Y, Zhao L, Mei H, Huang ZH, Zhang SL. Alterations of biliary biochemical constituents and cytokines in infantile hepatitis syndrome. World J Gastroenterol. 2006;12:7038–41.
21. Narayanaswamy B, Gonde C, Tredger JM, Hussain M, Vergani D, Davenport M. Serial circulating markers of inflammation in biliary atresia - evolution of the post-operative inflammatory process. Hepatology. 2007;46:180–7.