48uep6bbphidvals|361
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Various classifications of chronic gastritis have been described. Strickland and Mackay[1] first classified chronic gastritis into type A (autoimmune) and type B (environmental). Types A involves fundus-body and is associated with histamine fast achlorhydria, hypergastrinemia and severe vitamin B12 malabsorption. Parietal cell antibodies (PCA) and intrinsic factor antibodies (IFA) were present in the serum of 90% and 75% respectively. Type B gastritis involves the antrum with normal acid secretion or hypochlorhydria, normal serum gastrin and is associated with normal vitamin B12 absorption. PCA was present in 60% patients but IFA was absent in these patients. In 1973, Desai and Antia [2] proposed the earliest immunological classification of chronic gastritis from India, in which chronic gastritis was classified into 3 types- Type 1: absence of both PCA and IFA in serum (e.g. post operative or corrosive gastritis), Type 2: presence of PCA and absence of IFA (superficial or atrophic gastritis) and Type 3: presence of both PCA and IFA (Pernicious anaemia-PA). Since the discovery of H. pylori in 1984, it has been incorporated as an important etiological agent for chronic gastritis.[3] H. pylori gastritis has been known to be antral predominant which progresses to chronic atrophic gastritis, intestinal metaplasia and gastric adenocarcinoma.[4,5] Subsequently Sydney system of classification was described and was revised later.[6,7] It was based on topography, morphology and etiology. In this classification, chronic gastritis was broadly categorized as nonatrophic, atrophic and special forms. Atrophic gastritis was further sub-classified depending on etiology as autoimmune, where etiology is mainly antibodies (PCA and IFA) and questionable role of H. pylori. Another type of atrophic gastritis is, multifocal atrophic gastritis in which H. pylori is considered as the major etiological factor in conjunction with other environmental factors.
By now it has been established that H. pylori infection induces gastric autoimmunity by producing antibodies that cross reaction with gastric parietal cells.[8,9] H. pylori antibodies have been also reported in patients with PA.[10] In 1992 Desai and Amarapurkar[11] proposed a new classification of chronic gastritis taking into consideration, the role of autoimmunity. They specifically separated chronic atrophic gastritis with histamine fast achlorhydria from PA on the basis of absence or presence of IFA and concluded that the diagnosis of PA should be restricted to IFA positivity only. Those without IFA should be included under the category of chronic atrophic gastritis.With reference to the above concept, the aim of this study was to differentiate IFA positive from IFA negative chronic atrophic gastritis.
Methods
Fifty consecutive patients diagnosed as chronic atrophic gastritis on biopsy were included in the study over a period of 5 years from 2004 to 2009. Patients taking anti-secretory drugs, NSAIDS or with a history of ulcer disease, gastric and / or intestinal surgery or malignancy were excluded from study. All fifty patients included were evaluated clinically. History of associated autoimmune diseases, was also noted.
Routine hematological investigations were done with a special emphasis on hemoglobin and mean corpuscular volume (MCV). Biochemical tests, which reflect vitamin B12 malabsorption (serum LDH and serum B12) were undertaken. Vitamin B12 levels were performed using standard MEIA method. Fasting serum gastrin levels were also evaluated in all the patients by specific radioimmunoassay (RIA).{Normal range for the test values were: Hemoglobin = 12 to 14 gm%, MCV = 82 to 94 FL, serum LDH = 105 to 333 U/L, Vitamin B12 = 200 to 900 pg/ml, serum gastrin = < 100 pg / ml}.
All these patients were subjected to determination of parietal cell and intrinsic factor antibody using standard ELISA method (INOVA Diagnostics, San Diego, CA). PCA (IgA) >15 units / ml and IFA (IgG) > 1.1 were considered as positive. Multiple gastric biopsies were taken from body and antrum of stomach along the greater curvature. Biopsies were processed routinely and reported by experienced gastrointestinal pathologist. On histology, atrophy of gastric mucosa was defined as focal or complete oxyntic gland loss and / or their replacement by metaplastic pyloric or intestinal epithelium.[12] Histological features observed were, severity of gastritis (amount of inflammation), presence of intestinal metaplasia, endocrine cell hyperplasia, gastric carcinoid and H. pylori infection. H. pylori infection was diagnosed on routine staining. For ECL hyperplasia and gastric carcinoid, immunohistochemistry was done wherever necessary. Patients were divided into 2 main groups. Those which were IFA positive (Group A) and those IFA negative (Group B). The mean laboratory values and histological parameters were compared between the two groups.
Statistical evaluation
Data was expressed as mean values and / or number or total (percentage %). The differences between 2 groups were analysed using Students‘t’ test, Fisher’s exact test and Mann Whitney test as appropriate. Two tailed p values < 0.05 were considered as statistically significant.
Results
Of the total 50 patients, 18 were in group A (mean age 55.5+13 years, M:F 16:2) and 32 in group B (mean age 49.7+ years, M:F 25:7). PCA was positive in 40 (80 %) cases. In group A, two cases and in group B, eight cases were negative for PCA. Haemoglobin, MCV, LDH, vit B12 and serum gastrin levels were comparable in both groups. (Table 1). MCV and LDH values were high which were considered to be markers of Vit B12 deficiency. However there was no statistical difference in both the groups. Vit B12 < 200 pg/ml was considered as abnormal. Mean Vit B12 was 127.8 ± 114pg/ml and 112.7±69 pg/ ml in group A and B respectively. Mean serum gastrin level was higher in group A however there was no significant difference from group B. Seven (38.8 %) patients (2, type II diabetes mellitus; 3, thyroid disorders; 1, type II diabetes with thyroid disorder; and 1 had anti-endomysial antibody positivity) in group A and 8 (25 %) in group B (2, type II diabetes mellitus; 5, thyroid disorders; and 1, anti-endomycial antibody positivity) showed associated autoimmune diseases.
Considering histological parameters, (Table 2) intestinal metaplasia was present in 9 (50 %) and 11 (34.3 %), endocrine cell hyperplasia 5 (29.4 %) and 10 (31 %), gastric carcinoid 4 (23.5 %) and 9 (28 %) and H. pylori infection in 5 (29.4 %) and 5 (15.6 %), in group A and B respectively (Figure 1). None of these histological parameters showed statistically significant difference between the two groups (p > 0.05).
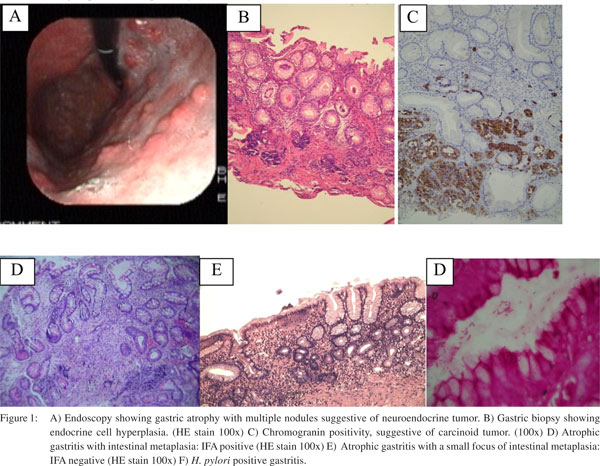
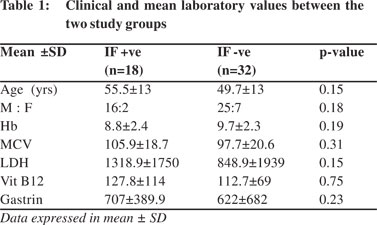
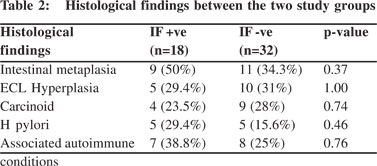
Discussion
Traditionally two main pathogenic mechanisms are considered responsible for development of atrophic gastritis. These are H. pylori related and autoimmune mechanisms.[13,14,15] H. pylori gastritis is antral predominant, with continued inflammation. There is development of hypo- or achlorhydria which facilitates migration of bacteria from antrum to body and eventually resulting into advanced corpus atrophy and intestinal metaplasia.[16] Once intestinal metaplasia sets in H. pylori disappears as they can not survive in the environment of metaplastic epithelium.
Another mechanism is of autoimmune gastritis which is characterized by presence of PCA and IFA antibodies and associated with other autoimmune diseases.[17,18,19] The pathological lesion of autoimmune gastritis is atrophic body gastritis, hypo- / achlorhydria and high serum gastrin levels (hypergastrinemia). Hypergastrinemia is a good stimulus for endocrine cell hyperplasia which can progress to development of carcinoids.[15, 20,21,22] PA is considered to be an end stage of autoimmune gastritis and is characterised by macrocytic anaemia, atrophic gastritis and presence of IFA.[23,24]
PCA has been known to be present in upto 60-90% cases of autoimmune gastritis, while IFA is found in 30-50% patients.[19,21,23] PCA are targeted against gastric H+/K+ ATPase. They can be seen in H. pylori related gastritis and also in upto 10% of normal healthy individuals.[25] Epidemiological data from western countries has shown that there is age related increase in prevalence of PCA from 2.5% in the 3rd decade to 12% in the 8th decade.[26,27] Hence PCA positivity is not specific for autoimmune gastritis but its presence may indicate atrophic damage to gastric body mucosa.
IFA is considered as a specific marker for PA and not for atrophic body gastritis without PA. IFA is present in upto 30- 50% patients of autoimmune gastritis but has not been reported in patients without atrophy. Hence IFA positivity is thought to be 100% specific for atrophic gastritis.[28] IFA titres may indicate atrophic damage to gastric body mucosa. Lahner et al[28] while analysing the role of PCA and IFA in atrophic gastritis with respect to cobalamin deficiency, found that IFA positivity was 27% in atrophic body gastritis, as compared to none in control population (non-atrophic gastritis patients). IFA positivity was seen not only in patients with PA, but also in patients with atrophic gastritis without PA, suggesting that IFA has 100% specificity and PPV in diagnosis of atrophic gastritis. However, in spite of high specificity, the prevalence of IFA antibodies in atrophic gastritis is low. Hence in this study we tried to differentiate clinical, haematological, biochemical features between IFA positive and negative chronic atrophic gastritis. In the present study none of the hematological and biochemical features showed statistically significant difference between the two groups. There was also no difference in intestinal metaplasia, endocrine cell hyperplasia, carcinoid and H. pylori infection between group A and B. Similar results have been obtained by Annibale et al[29] while evaluating gastric autoimmune phenomenon in atrophic gastritis patients. They have compared 49 IFA positive and 91 IFA negative patients and did not find significant difference in demographic, clinical, biochemical and histological features. Another important finding from their study was positive correlation between IFA levels and histological score of atrophic gastritis. They have suggested that progression of oxyntic mucosal damage leads to production of IFA. This damage can be due to H. pylori infection. At an early stage of H. pylori infection, when the organisms are detectable on tissue sections, IFA is significantly lower than that in patients with long standing infection, in whom only immunological memory or no sign of infection is detectable.
As H. pylori has been shown to modulate the immune response, simultaneous infection with this pathogen has masked the natural history of H. pylori gastritis.[30] Endocrine cell hyperplasia and gastric carcinoids were thought to be common in autoimmune gastritis, however H. pylori associated chronic atrophic gastritis exhibit a gradual reduction in acid secretion and can develop endocrine cell hyperplasia. Hence, this data suggest that autoimmune origin of atrophic body gastritis is not different from non-autoimmune atrophic body gastritis. It may be the spectrum of one disease, where H. pylori may be responsible for initiating the process.
References
1. Strickland RG, Mackey IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973;18:426.
2. Desai HG, Antia FP. A second look at chronic gastritis. A new classification. Indian J Med Sci. 1973;27:217.
3. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5.
4. Prabhu SR, Amarapurkar AD, Amarapurkar DN. Role of Helicobacter pylori in gastric carcinoma. Natl Med J India. 1995;8:58–60.
5. Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60:3–21.
6. Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209–22.
7. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
8. Presotto F, Sabini B, Cecchetto A, Plebani M, De Lazzari F, Pedini B, et al. Helicobacter pylori infection and gastric autoimmune diseases: is there a link? Helicobacter. 2003;8:578–84.
9. Bergman MP, Vandenbroucke-Grauls CM, Appelmelk BJ, D’Elios MM, Amedei A, Azzurri A, et al. The story so far: Helicobacter pylori and gastric autoimmunity. Int Rev Immunol. 2005;24:63–91.
10. Desai HG, Gupte PA. Helicobacter pylori link to pernicious anaemia. J Assoc Physicians India. 2007;55:857–9.
11. Desai HG, Amarapurkar DN. New look at chronic gastritis. J Clin Gastroenterol. 1992;14:278–80.
12. Genta RM. Helicobacter pylori, inflammation, mucosal damage, and apoptosis: pathogenesis and definition of gastric atrophy. Gastroenterology. 1997;113:S51–5.
13. El-Zimaity H. Gastritis and gastric atrophy. Curr Opin Gastroenterol. 2008;24:682–6.
14. Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2003;109,138–43.
15. Torbenson M, Abraham SC, Boitnott J, Yardley JH, Wu TT. Autoimmune gastritis: distinct histological and immunohistochemical findings before complete loss of oxyntic glands. Mod Pathol. 2002;15:102–9.
16. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process - first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–40.
17. Irvine WI. The association of atrophic gastritis with autoimmune thyroid disease. Clin Endocrinol Metab. 1975;4:351.
18. Hershko C, Skikne B. Pathogenesis and management of iron deficiency anaemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009;46:339–50.
19. De Block CE, De Leeuw IH, Bogers JJ, Pelckmans PA, Ieven MM, Van Marck EA, et al. Autoimmune gastropathy in type 1 diabetic patients with parietal cell antibodies: histological and clinical findings. Diabetes Care. 2003;26:82–8.
20. Korman MG, Strickland RG, Hansky J. Serum gastrin in chronic gastritis. Br Med J. 1971;2:16–8.
21. Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anaemia. Gastroenterology. 1985;88:638–48.
22. Borch K. Atrophic gastritis and gastric carcinoid tumours. Ann Med. 1989;21:291–7.
23. Toh BH, Alderuccio F. Pernicious anaemia. Autoimmunity. 2004;37:357–61.
24. Desai HG, Antia FP. Vitamin B 12 malabsorption due to intrinsic factor deficiency in Indian subjects. Blood. 1972;40:747–53.
25. Desai HG, Dighe PK, Borkar AV. Parietal cell and intrinsic-factor antibodies in Indian subjects. Scand J Gastroenterol. 1968;3:321–6.
26. Whittingham S, Mackay IR. Pernicious anaemia and gastric atrophy. In: Rose NR, Mackay IR, editors. The autoimmune diseases. New York: Academic Press; 1985.p.243–266.
27. Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–43.
28. Lahner E, Norman GL, Severi C, Encabo S, Shums Z, Vannella L, et al. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol. 2009;104:2071–9.
29. Annibale B, Lahner E, Negrini R, Baccini F, Bordi C, Monarca B, et al. Lack of specific association between gastric autoimmunity hallmarks and clinical presentations of atrophic body gastritis. World J Gastroenterol. 2005;11:5351–7.
30. Annibale B, Lahner E, Bordi C. Role of Helicobacter pylori infection in pernicious anaemia. Dig Liver Dis. 2000;32:756–62.