|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
|
|
|
Gourdas Choudhuri, CP Lakshmi, Amit Goel
Professor & Head Department of Gastroenterology
Sanjay Gandhi Postgrdaduate
Institute of Medical Sciences,
Rai Bareily Road, Lucknow-226014,
Uttar Pradesh, India
Corresponding Author:
Dr Gourdas Choudhuri
E mail: gourdas@satyam.net.in
DOI:
http://dx.doi.org/
Abstract
Pancreatic endocrine insufficiency secondary to destruction of acinar cells is a well known complication of chronic pancreatitis (CP). Of all patients with diabetes mellitus, 0.5-1% is secondary to CP. The frequency of occurrence of diabetes in CP is about 40-60%. This figure varies according to the aetiology, extent of calcification, and duration of the disease. Pancreatic diabetes is more commonly associated with alcoholic and tropical calcific pancreatitis of long duration. The pathophysiology of pancreatic diabetes is related to beta cell failure and reduced insulin secretory capacity. The development of pancreatic diabetes calls for careful evaluation and management to prevent long term complications. Pancreatic cancer is a known complication of chronic pancreatitis and sometimes manifests with new onset diabetes.
As destruction of pancreatic tissue in CP leads to depletion of both insulin and glucagonproducing cells of the islets of Langherhans, pancreatic diabetics are usually not prone to ketoacidosis. A trial of oral hypoglycemic agents followed by insulin therapy when the need arises has been the line of management thus far in these patients. This review focuses on the prevalence, unique pathophysiological aspects, clinical features and special issues in the management of diabetes secondary to chronic pancreatitis.
|
48uep6bbphidvals|199 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext The first description of an association between pancreatic calcification and diabetes dates back to 1788, in the form of a monograph in the London Medical Journal by Cawley et al.[1] Studies in the ensuing centuries firmly established the relationship between chronic pancreatitis (CP) and diabetes. In this article on pancreatic diabetes we shall review its prevalence, unique pathophysiological aspects, clinical features and special issues in its management.
Definition
Diabetes mellitus (DM) implies a group of metabolic disorders sharing the phenotype of hyperglycemia. The World Health Organisation, in 1997, issued criteria for its diagnosis, which include symptoms of diabetes plus random blood glucose concentration exceeding 200 mg/dL or fasting plasma glucose more than 126 mg/dL or a two-hour plasma glucose more than 200 mg/dL during an oral glucose tolerance test. DM is classified on the basis of the pathogenic process that leads to the hyperglycemia. In the current classification, type 3 DM includes diabetes due to various secondary causes, and DM secondary to pancreatic exocrine dysfunction is placed in the type 3c subcategory.[2] Older terminology like fibrocalculous pancreatic diabetes has been removed from the WHO dictionary.
Epidemiology
Patients with pancreatic diabetes (PD) form a minor portion of all diabetics. Western literature reveals that PD accounts for 0.5-1.2% of all patients with DM.[3] In the Asia-Pacific region, a Japanese study showed that 1.69% of 17,500 diabetic patients had pancreatic diabetes.[4] Indian data are also available; a population-based study from Chennai showed that fibrocalculous pancreatic diabetes constituted 0.36% of all cases of self-reported diabetics.[5]
Diabetes in CP and factors affecting its incidence
The frequency of diabetes in CP ranges from 40-60%. This figure varies depending on the aetiology of the chronic pancreatitis, the extent of calcification and the duration of disease. Amongst the various causes of CP, alcoholic chronic pancreatitis (ACP) and tropical calcific pancreatitis (TCP) have the maximum frequency of PD. These have been the subject of several studies, which have been summarised in Tables 1 and 2.
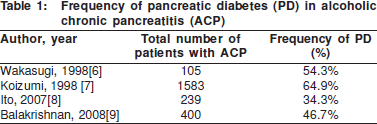
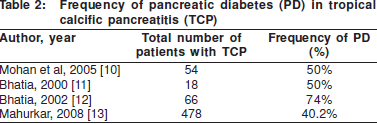
The association between the presence of pancreatic calcification and the onset of PD was noted as early as 1975. Calcification is an indicator of long standing and advanced disease, and probably correlates well with pancreatic tissue loss. In a series of 1235 patients with CP, Banks et al1 found that 98% of patients with pancreatic calcification developed diabetes over a follow up period of 5 years, whilst only 75% of CP patients without calcification developed PD. Other studies also showed that calcification was significantly associated with the PD.[14] Recent data shows that the risk of diabetes increases 1.32 fold after the onset of calcification.[8]
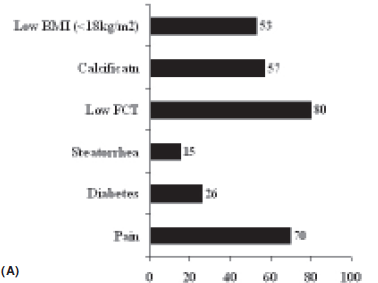
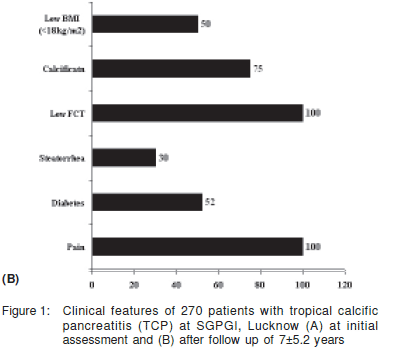
There is a strong correlation between the duration of CP and the incidence of PD. Malka et al[14] showed that the cumulative rates of appearance of PD calculated since the onset of disease were 50% and 83 % at 10 and 25 years, respectively. Japanese authors have shown that in a cohort of 656 patients with chronic pancreatitis, the presence of diabetes increased from 35.1% in 1994 to 50.4% after 8 years. In this study the incidence was highest in patients with ACP, especially in those who continued to consume alcohol.[8] Mohan et al followed up 54 TCP patients without diabetes and found similar results; 50% of their patients developed DM on 5 year follow up. The median time to development of diabetes was 9.6 years after the diagnosis of TCP.[10] In our series of 270 patients, PD was present in 26% at presentation with its frequency increasing to 52% over 7.5 years of follow up. (Figure 1).[15]
Aetiology
An array of pathological conditions leading to exocrine pancreatic tissue loss, may lead to PD. Common conditions include acute as well as chronic pancreatitis, pancreatectomy, pancreatic neoplasm, cystic fibrosis, and hemochromatosis. Among all the above causes of pancreatic insufficiency, chronic pancreatitis (CP) is the most common. It is often consequent to heavy prolonged alcohol consumption, genetic disorders as in hereditary pancreatitis or as a syndromic form of unknown aetiology occuring in young people in the tropics and associated with stones in the pancreatic duct as in tropical chronic pancreatitis (TCP). Symptomatic overt diabetes is more likely to complicate TCP, ACP (as discussed above) and also autoimmune pancreatitis (AIP). Its incidence is lower in idiopathic chronic pancreatitis and uncommon in CP due to other causes such as biliary, traumatic or post surgical.
Pathophysiology
Islets of Langerhans are resistant to destruction early in the course of CP, but as the disease advances, both insulinproducing beta cells and glucagon-producing alpha cells are progressively destroyed. The loss of insulin secretory capacity leads to the clinical syndrome of pancreatic diabetes. In a study undertaken to assess beta cell function and insulin sensitivity in TCP patients, beta cell function was found to be significantly lower in TCP patients with PD compared to controls, while insulin sensitivity was similar in both groups. These findings indicate that the diabetes in patients with TCP develops due to a loss of beta cell function, and not due to insulin resistance.[16]
In parallel to the loss of insulin secretion, glucagon secretory capacity also declines progressively in these patients. The clinical importance of this lies in the fact that if hypoglycemia develops during therapy in these patients, the effect is prolonged, as the compensatory glucagon secretory response to restore euglycemia is blunted. Catecholamine secretion is preserved (yet blunted) in PD and other forms of secondary diabetes. Pancreatic polypeptide secretion is also reduced in parallel with the fall in other endocrine functions of the pancreas. This in turn leads to loss of hepatic insulin receptor expression and hepatic insulin receptor dysfunction.[17] Exocrine pancreatic insufficiency also leads to diminished incretin release. Concomitant alcohol abuse in these patients often adds several associated problems such as hepatic disease, direct toxic effects of alcohol and malnutrition.
The role of genes in the pathophysiology of PD has been the subject of several recent investigations. The SPINK 1 (serine protease inhibitor, Kazal type 1) gene regulates inactivation of excess trypsin produced by pancreatic acinar cells, and thus prevents autolysis and pancreatic damage; its mutation resulting in an N34S variant of SPINK1 has been shown as a strong risk factor for TCP, with 44% of TCP patients demonstrating the mutation compared to 2.2% of controls.[12] The frequency of this mutation was similar in Indian TCP patients with or without PD. In a study from Bangladesh, apart from observing similar findings in patients with TCP, type 2 diabetics also showed a higher frequency of this mutation.[18]
Hereditary pancreatitis is a condition characterised by recurrent episodes of pancreatic inflammation starting at a very young age, resulting in damage to the gland and development of diabetes. It results from an R122 H substitution in cationic trypsinogen gene (PRSS 1) at the autolysis site resulting in a “gain of function” mutation because prematurely activated trypsin cannot be inactivated by autolysis. This genetic mutation was however not found in patients of TCP. .[12]
Studies have tried to assess the correlation between pancreatic endocrine and exocrine insufficiency in the setting of TCP. A study from our institute in 1995 showed that of the 20 TCP patients with PD, 13 (65%) had exocrine pancreatic insufficiency as evidenced by a low faecal chymotrypsin level.[19] Mohan et al[15] have also shown that patients of TCP who develop PD on follow up have lower faecal chymotrypsin levels when compared to those who do not develop diabetes.
Pathology
On histopathology, the findings in PD include moderate to severe atrophy of the pancreas, fibrosis of the parenchyma and degeneration of the ducts. Nesidioblastosis may also be present. Immunohistochemical studies have shown a fall in the number of both insulin-producing beta cells and glucagonproducing alpha cells, along with reduced positivity for pancreatic polypeptide and somatostatin.[20] The reduction, but partial preservation of insulin positivity is consistent with the ketosis resistance shown by patients with PD. In addition, the levels of islet amyloid polypeptide (amylin) are elevated in CP patients with diabetes; similar to those with type 2 DM.
Clinical features
The clinical features of PD are similar to those of diabetes due to other causes. Predominant symptoms are polyuria, polydipsia and polyphagia which vary in onset. The associated features of CP like a history of pancreatic pain, steatorrhea, etc help in making a diagnosis. Weight loss may be a prominent feature in these patients. This could be due to the diabetic state per se or due to other causes like chronic abdominal pain restricting food intake, steatorrhea, small bowel bacterial overgrowth or underlying pancreatic malignancy.
In our experience with 270 patients of CP on follow up in our Pancreas Clinic, the frequency of each clinical feature has been summarised in Figure 1.[21] Overall, 53% of our patients with CP had a low body mass index, and 26% had diabetes at presentation. Abdominal pain and steatorrhea were present in 70% and 15%, respectively.
Natural history
High blood sugar levels found in almost 50% of patients with acute pancreatitis, usually resolve following recovery. Patients with severe acute pancreatitis are at risk of developing pancreatic diabetes on follow up. Studies have shown that 26% to 39% of patients with severe acute pancreatitis develop diabetes later on;[22,23] the figure rises to 75% in those who need to undergo necrosectemy. [23] The majority of these patients are not insulin dependent. The risk is more in alcoholic pancreatitis, especially in patients with ongoing alcohol abuse. [22] If the patient continues to take alcohol and has recurrent attacks of acute pancreatitis, glucose tolerance becomes progressively impaired with each attack, leading to the development of frank diabetes over time.
The risk of diabetes in patients with CP increases with the increasing duration of pancreatitis. The degree of endocrine failure worsens with time. A study done in our institute showed that endogenous insulin secretion assessed by estimating C peptide levels in our PD patients, declined serially withincreasing duration of the disease.[24]
A large Indian study tracking complications of diabetes showed that the prevalence of retinopathy, nephropathy, neuropathy, and peripheral vascular disease was similar among PD and type 2 diabetics, but the prevalence of coronary artery disease was lower in PD patients.[25] In a cohort of 370 patients with PD, Mohan et al found that the median survival after the onset of diabetes was 25 years, with diabetic nephropathy as the major cause of death in these patients. 80% of the patients were alive at 35 years after the onset of the first episode of pain.[26]
Pancreatic cancer and diabetes
The diabetes in pancreatic cancer is typically described as new onset DM (<2 years duration). The frequency of DM in pancreatic cancer reportedly ranges from 4% to 64%. This wide range is partly due to the different criteria used in the diagnosis of diabetes in various series. Pannala et al[27] analysed the data of 512 patients with pancreatic cancer and found that 47% of them had DM according to the WHO criteria. The same study also showed that patients of pancreatic cancer who had DM were older, had higher body mass index and a greater frequency of family history of DM compared to those without DM. After pancreaticoduodenectomy, while DM resolved in 57% with new onset DM, its prevalence remained the same in patients with long-standing DM.
Pancreatic cancer is one of the dreaded complications of TCP. On follow up of 185 patients with TCP, it was found that 25% of all deaths in the cohort were due to pancreatic cancer, with a median age of onset of 45.6 years, considerably younger than that of the Western population. The relative risk of pancreatic cancer in TCP is about 5.95.[28] One study also found that 73% of patients with TCP and pancreatic carcinoma had PD, a figure much higher than the usual reported incidence of PD in CP.[29]
Treatment of PD
The hyperglycemia of acute pancreatitis is transient and asymptomatic. During the acute phase of pancreatitis, frequent monitoring of the blood sugar level is usually all that is needed, with occasional use of short acting insulin to control hyperglycemia and ketosis. During insulin therapy, blood sugars should be monitored frequently to avoid precipitous fall in blood sugar levels. A glucose tolerance test may be done after three months of the attack to assess the status of endocrine function.
The clinician should be cautious in managing patients with type 3c diabetes.[30] The diabetes in these patients is very brittle and awareness of hypoglycemia is poor. Ketoacidosis is distinctly uncommon as insulin secretion is not completely destroyed in patients with CP. It is suggested that a conservative therapeutic approach be taken in these patients as they often show extreme sensitivity to insulin therapy and develop prolonged hypoglycemia.
Some patients may be carefully initiated on oral hypoglycemic agents, a sulfonylurea, thiazolidinedione or metformin. Sulfonylureas may be associated with a risk of severe and prolonged hypoglycemia and careful blood sugar monitoring is necessary. Metformin should be avoided in patients with ongoing alcohol abuse due to the risk of lactic acidosis. Alpha-glycosidase inhibitors might aggravate the existing GI complaints of exocrine insufficiency. In patients who do not respond satisfactorily to oral hypoglycemic agents, insulin is needed, but insulin requirements are generally lower than those of patients with type 1 DM.
Even though a significant proportion of patients can be treated with dietary modification and oral antidiabetic drugs, without using insulin, controlled clinical trials about the effects and risks of treatment with oral antidiabetic agents and insulin in type 3c diabetes mellitus are lacking. The influence of pancreatic enzyme replacement therapy on glucose metabolism in insulin-treated patients with exocrine insufficiency also remains controversial.[30,31]
In patients with alcohol-related CP, the importance of alcohol abstinence should be emphasised and de-addiction therapy and counselling started along with the treatment of PD. Patients with PD complicating autoimmune pancreatitis should be started on glucocorticoids along with insulin therapy for PD. Some authors have even shown reversal of the diabetic state following successful glucocorticoid therapy for autoimmune pancreatitis.[32]
Surgical or endoscopic decompression of the pancreatic duct has a significant role in reducing pain in patients with CP but not in the management of the diabetic state. In a prospective study at our centre, we followed up 14 patients after surgical or endoscopic ductal decompression and found that there was no difference in beta cell function (as measured by C peptide levels) or exocrine function although there was significant improvement in pain.[31]
We can conclude that management of PD is mainly medical, includes a trial of oral hypoglycemic agents, and sometimes requires insulin if there is inadequate response to oral drugs. Management of pain, steatorrhea and other manifestations of associated CP should also be of paramount importance.
Conclusions
To sum up, pancreatic diabetes accounts for 0.5-1% of all patients with DM and develops in the course of chronic pancreatitis in 50% of patients. Its pathophysiology is unique and it is brittle and is resistant to ketosis, but can be well managed with diet and OHAs, with insulin therapy playing a role later in the course of diease.
References
1. Simmy Banks, Mark IN, Vinik AN. Clinical and hormonal aspects of pancreatic diabetes. Am J Gastroenterol. 1975;64:13–22.
2. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97.
3. Philip D Hardt, Mathias D Brendel, Hans U Kloer, Reinhard G Bretzel. Is Pancreatic Diabetes (Type 3c Diabetes) Underdiagnosed and Misdiagnosed? Diabetes Care. 2008;31(Suppl. 2):S165–S169.
4. Okuno G, Oki A, Kawakami F, Doi K, Baba S. Prevalence and clinical features of diabetes mellitus secondary to chronic pancreatitis in Japan; a study by questionnaire. Diabetes Res Clin Pract. 1990;10:65–71.
5. Mohan V, Farooq S, Deepa M. Prevalence of Fibrocalculous Pancreatic Diabetes in Chennai in South India. JOP. 2008;9:489–92.
6. Wakasugi H, Funakoshi A, Iguchi H. Clinical assessment of pancreatic diabetes caused by chronic pancreatitis. J Gastroenterol. 1998;33:254–9.
7. Koizumi M, Yoshida Y, Abe N, Shimosegawa T, Toyota T. Pancreatic Diabetes in Japan. Pancreas. 1998;16:385–91.
8. Ito T, Otsuki M, Itoi T, Shimosegawa T, Funakoshi A, Shiratori K. Pancreatic diabetes in a follow-up survey of chronic pancreatitis in Japan. J Gastroenterol. 2007;42:291–7.
9. Balakrishnan V, Unnikrishnan AG, Thomas V, Choudhuri G, Veeraraju P, Singh SP, et al. Chronic Pancreatitis. A Prospective Nationwide Study of 1,086 Subjects from India. JOP. 2008;9:593–600.
10. Mohan V, Barman KK, Rajan VS, Chari ST, Deepa R. Natural history of endocrine failure in tropical chronic pancreatitis: A longitudinal follow-up study. J Gastroenterol Hepatol. 2005;20:1927–34.
11. Bhatia E, Durie P, Zielenski J, Lam D, Sikora SS, Choudhuri G, Tsui LC. Mutations in the cystic fibrosis transmembrane regulator gene in patients with tropical calcific pancreatitis. Am J Gastroenterol. 2000;95:3658–9.
12. Bhatia E, Choudhuri G, Sikora SS, Landt O, Kage A, Becker M, et al. Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations. Gastroenterology. 2002;123:1020–5
13. Mahurkar S, Bhaskar S, Reddy DN, Prakash S, Rao GV, Singh SP, Thomas V, et al. TCF7L2 gene polymorphisms do not predict susceptibility to diabetes in tropical calcific pancreatitis but may interact with SPINK1 and CTSB mutations in predicting diabetes. BMC Med Genet. 2008;9:80.
14. Malka D, Hammel P, Sauvanet A, Rufat P, O’Toole D, Bardet P, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119:1324–32.
15. Chodhuri G, Bhatia E, Sikora SS, Alexander G. Tropical pancreatitis in North India. In: V Balakrishnan, Harishkumar, S Sudhakaran, AG Unnikrishnan. Chronic pancreatitis and pancreatic diabetes in India. 2006,55–62.
16. Mehrotra RN, Bhatia E, Choudhuri G. Beta-cell function and insulin sensitivity in tropical calcific pancreatitis from north India. Metabolism. 1997;46:441–4.
17. Andersen DK. Mechanisms and emerging treatments of the metabolic complications of chronic pancreatitis. Pancreas. 2007;35:1–15.
18. Schneider A, Suman A, Rossi L, Barmada MM, Beglinger C, Parvin S, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002;123:1026–30.
19. Bhatia E, Baijal SS, Kumar KR, Choudhuri G. Exocrine pancreatic and beta-cell function in malnutrition-related diabetes among north Indians. Diabetes Care. 1995;18:1174–8.
20. Govindarajan M, Mohan V, Deepa R, Ashok S, Pitchumoni CS. Histopathology and immunohistochemistry of pancreatic islets in? brocalculous pancreatic diabetes. Diabetes Res Clin Pract. 2001;51:29–38.
21. Choudhuri G, Singh D, Lakshmi CP. Tropical pancreatitis. Ceylon Med J. 2008;53:4–6.
22. Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:397–402.
23. Sabater L, Pareja E, Aparisi L, Calvete J, Camps B, Sastre J, et al. Pancreatic function after severe acute biliary pancreatitis: the role of necrosectomy. Pancreas. 2004;28:65–8.
24. Mittal N, Mehrotra R, Agarwal G, Rajeswari, Choudhuri G, Sikora S, Bhatia E. The clinical spectrum of fibrocalculous pancreatic diabetes in north India. Natl Med J India. 2002;15:327–31.
25. Barman KK, Padmanabhan M, Premalatha G, Deepa R, Rema M, Mohan V. Prevalence of diabetic complications in fibrocalculous pancreatic diabetic patients and type 2 diabetic patients: A crosssectional comparative study. Journal of Diabetes and Its Complications. 2004;18:264–70.
26. Mohan V, Premalatha G, Padma A, Chari ST, Pitchumoni CS. Fibrocalculous pancreatic diabetes. Long-term survival analysis. Diabetes Care. 1996;19:1274–8.
27. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and Clinical Pro?le of Pancreatic Cancer–Associated Diabetes Mellitus. Gastroenterology. 2008;134:981–7.
28. Chari ST, Mohan V, Pitchumoni CS, Viswanathan M, Madanagopalan N, Lowenfels AB. Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas. 1994;9:62–6.
29. Augustine P, Ramesh H. Is tropical pancreatitis premalignant? Am J Gastroenterol. 1992;87:1005–8.
30. Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes(type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care. 2008;31 Suppl 2:S165–9.
31. Agarwal G, Sikora SS, Choudhuri G, Bhatia E. Prospective study of pancreatic b-cell and exocrine function following duct decompression in tropical calcific pancreatitis. World J Surg. 2002;26:171–5.
32. Tanaka S, Kobayashi T, Nakanishi K, Okubo M, Murase T, Hashimoto M, et al. Corticosteroid-responsive diabetes mellitus associated with autoimmune pancreatitis. Lancet. 2000;356:910–1.
|
|
|
 |
|
|