|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
lactose malabsorption, functional bowel disease, lactase deficiency |
|
|
Uday C Ghoshal, Sunil Kumar, Dipti Chourasia, Asha Misra
Department of Gastroenterology,
Sanjay Gandhi Post Graduate
Institute of Medical Sciences,
Lucknow – 226014, India
Corresponding Author:
Dr. Uday C Ghoshal
Email: udayghoshal@gmail.com
DOI:
http://dx.doi.org/
Abstract
Background: We compared the lactose hydrogen breath (lactose HBT) and lactose tolerance tests (LTT) in their ability to diagnose lactose malabsorption (LM).
Methods: Fasting and post-lactose (50 g) breath hydrogen and blood sugar were tested in patients with irritable bowel syndrome (IBS). Persistent rise in breath hydrogen by 20 ppm and failure of blood sugar to rise by >20 mg/dL above basal level, were considered positive lactose HBT and LTT, respectively. Symptoms of diarrhoea, bloating, abdominal pain and flatulence were noted.
Results: Of 203 patients, 11 demonstrated high basal breath hydrogen and hence, 192 (age 37±14 years, 134 male) were included in the study. 125 (65%) and 137 (71%) were lactose HBT and LTT positive, respectively. 102/125 lactose HBT positive patients were LTT positive and 35/67 lactose HBT negative patients were LTT positive. 62/192 (32%) developed symptoms following lactose ingestion, which tended to be more in the LTT positive (49/137, 36% vs. 13/55, 24% p=0.07) but not in the lactose HBT positive patients (44/125, 35% vs. 18/67, 27% p=0.2). Peak breath hydrogen was higher (38±37 vs. 66±43; p<0.01) in LTT positive than negative patients. Peak level of breath hydrogen inversely correlated (58±43 vs. 10±23; p<0.001) with change in blood glucose following lactose ingestion.
Conclusions: Positive LTT is associated with a higher breath hydrogen score than negative LTT. There was a trend towards more frequent symptom development following lactose load in LTT positive but not in lactose HBT positive patients. LTT is an easy and efficient test for diagnosis of LM.
|
48uep6bbphidvals|201 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Lactose malabsorption (LM) is a common problem in the adultpopulation all over the world in general, particularly in tropical countries.[1,2,3] There are several methods to evaluate LM like lactose hydrogen breath test (lactose HBT), lactose tolerance test (LTT), genetic test[4] and estimation of lactase level in intestinal biopsy.[5,6] Of these, the last two tests are either invasive or technically demanding and are not widely available. Hence, lactose HBT and LTT are commonly used to diagnose LM. An abnormal LTT denotes failure of the blood glucose to rise thirty minutes after ingestion of a 50 g lactose load, due to lack of digestion of lactose by intestinal lactase to form glucose and galactose. In contrast, abnormal lactose HBT is associated with an increase in breath hydrogen by 20 ppm above the basal level after ingestion of 50 g lactose due to bacterial fermentation of unabsorbed lactose within the intestinal lumen. While LTT is expected to be more specific for LM, abnormal lactose HBT can also result from other causes such as small intestinal bacterial overgrowth (SIBO).[7] Furthermore, though LTT requires estimation of blood glucose of lactose, lactose HBT requires prolonged monitoring of breath hydrogen; there are contradictory data on how long breath hydrogen needs to be monitored.[6,8,9,10] Data concerning the concordance between LTT and lactose HBT in diagnosing LM in the adult are scanty, contradictory and derived from a small sample size.[11,12]
Symptoms of irritable bowel syndrome (IBS), a functional gastrointestinal disorder include irregular bowel movement, latulence and abdominal fullness and abdominal pain and discomfort; these symptoms can also result from LM[13,14] and are often associated with LM in India.[3] A previous study showed that patients with IBS more often experienced symptoms following a lactose load compared to healthy subjects.[3] This might have been related to distension of the gut by hydrogen gas produced on bacterial fermentation of malabsorbed lactose.[3] Peak level of breath hydrogen following 50 g lactose load was comparable between patients of IBS and healthy subjects in that study.[3] However, the more the amount of hydrogen production in response to a given dose of lactose; greater would be the symptoms of flatulence and bloating. If our hypothesis that an abnormal LTT denotes more severe LM is correct, then patients with abnormal LTT are expected to have higher hydrogen production in response to a given dose of lactose and hence more frequent symptoms of bloating and flatulence.
Accordingly, we undertook a study with the following aims, (a) to study the concordance between lactose HBT and LTT in patients with IBS, (b) to study the level of breath hydrogen in relation to positive LTT, (c) to study the relationship between symptoms following lactose load and maximum level of breath hydrogen.
Methods
Patients: Patients with IBS, diagnosed according to Rome II criteria, referred to Gastrointestinal Pathophysiology and Motility Laboratory of a tertiary referral centre for evaluation of LM from December 2006 to March 2008, were included in the study. Each patient underwent other diagnostic investigations such as microscopic examination of the stools and testing for occult blood and hemogram. The Institutional Ethics Committee approved the study protocol and consent was obtained from each patient.
Hydrogen breath tests: Lactose HBT and glucose hydrogen breath test (GHBT) were performed using a breath gas analyser (Lactoscreen® H2 breath tester, Hoek Loos, Amsterdam, Netherlands). Basal breath specimens were obtained after an overnight fast; the subjects avoided slowly absorbed carbohydrates (bread, potato, corn) and fibre the previous evening to avoid delayed excretion of hydrogen in the breath.[15] Cigarette smoking and physical exercise were not permitted for 2 hours before and during the test, to prevent hyperventilation and consequent changes in breath hydrogen content.16 Each subject was asked to brush her/his teeth, and to rinse her/his mouth with an antiseptic wash followed by tap water, to eliminate an early hydrogen peak due to action of oralbacteria on test sugars.[17] Patients were asked to hold their breath for 15 seconds before blowing into the collection tube. An average of four values was taken as the basal breath hydrogen level. If the basal breath hydrogen value was >20 ppm on at least two days despite adequate preparation, performance of breath test was considered to have failed and the subject was excluded from the study. If the basal breath hydrogen was not high, then the patient was asked to ingest 50 g of lactose dissolved in 200 mL water for lactose HBT and 100 g glucose in 200 mL water for GHBT. Thereafter, breath hydrogen was estimated every 15 minutes for 3 to 4 hours. An increase in hydrogen excretion (ppm) following lactose or glucose administration was calculated by subtracting the basal value from the highest value of hydrogen excretion obtained. Persistent rise in breath hydrogen by 20 ppm above the basal level (at least two consecutive readings) following lactose ingestion and 12 ppm above the basal level following glucose administration was considered positive for lactose HBT and GHBT, respectively.3 Subjective increase of symptoms like diarrhoea, bloating sensation, abdominal pain or flatulence during the lactose HBT was also noted.
Each patient also underwent LTT; blood sugar was estimated with a glucometer (One touch Sure Step, Life scan, USA) using compatible glucostix, in the fasting state and 30 minutes after ingestion of lactose during lactose HBT by finger prick. Failure of the blood sugar to rise by >20 mg/dL above the fasting level 30 minutes after lactose ingestion was diagnostic of abnormal LTT.[3]
Statistical analysis: Continuous and categorical variables were compared using the unpaired t and Chi-squared tests, respectively. Two groups of continuous variables were correlated with Spearman correlation. A p value less than 0.05 was considered significant.
Results
Patients: Of the 203 patients screened, 192 (age: 37 ± 14 years, 134 male) were included in the study as the others demonstrated increased basal breath hydrogen levels whilst screening. All patients had symptoms of abdominal discomfort, irregular bowel habit, feeling of incomplete evacuation and the presence of mucus in stools. None of them had alarm symptoms including weight loss. Stool occult blood was negative in all patients.
Results of lactose hydrogen breath test and LTT: Of 203 patients, 11 (5.4%) had high basal breath hydrogen and hence excluded from further investigations. One hundred ninety-two patients were evaluated for LM using lactose HBT and LTT. Of them, 125 (65%) and 137 (71%) were lactose HBT and LTT positive, respectively. The frequency of positive lactose HBT (88/134, 66% male vs. 37/58, 64% female) and LTT (95/134, 71% male vs. 42/58, 72% female) was comparable in male and female patients. 62/192, 32% developed symptoms following lactose load. There was a trend towards development of symptoms following lactose ingestion in patients who were LTT positive (49/137, 36% vs. 13/55, 24% p=0.07) but not in those who were lactose HBT positive (44/125, 35% vs. 18/67, 27% p=0.2).
Relationship between age and results of lactose hydrogen breath test and LTT: The mean age of patients who were positive for lactose HBT was comparable to those who were negative (37±15 vs. 36±13, respectively). Similarly, patients with positive response to LTT were also comparable in age to those with negative test results (36±14 vs. 38±14 respectively).
Concordance between lactose hydrogen breath test and LTT: Of 137 patients who were LTT positive, 102 (74%) were lactose HBT positive. Of 55 patients who were LTT negative, 32 (58%) were lactose HBT negative. Proportion of agreement was 0.53 (kappa 0.62).
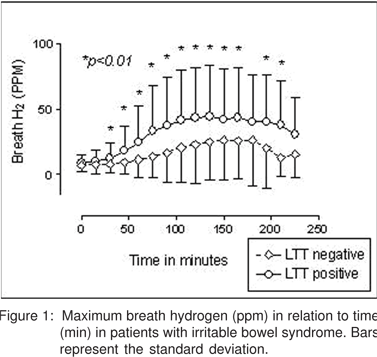
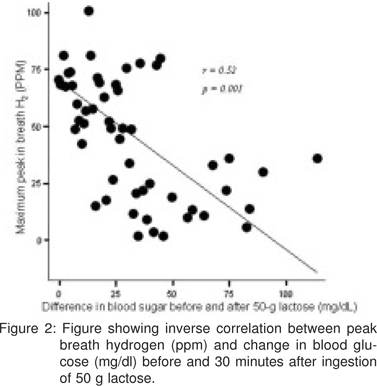
Level of breath hydrogen in relation to result of LTT: Peak level of hydrogen excreted in the breath was higher among LTT positive patients than LTT negative. (Figure 1) The peak level of hydrogen excreted in the breath inversely correlated with the change in blood glucose level between fasting and 30 minutes after ingestion of 50 g lactose. Figure 2)
Time to peak for lactose hydrogen breath test: Time to rise in breath hydrogen level 20 ppm above basal following lactose ingestion in 125 patients who were lactose HBT positive was 90.8± 44.4 minutes (range 15-195 minutes).
Result of glucose hydrogen breath test: Of 192 patients 115 underwent GHBT. 10/115 (8.7%) patients were positive to GHBT. Frequency of positive GHBT was comparable amongst lactose HBT positive and LTT positive patients (7/10, 70% vs. 8/10, 80%).
Discussion
The present study showed that LTT more often diagnosed LM than lactose HBT in patients with IBS. There was a rend towards development of symptoms following a lactose load in patients who were LTT positive but not in those who were lactose HBT positive. LTT positive patients produced a higher level of breath hydrogen throughout the ecording period compared to those who were LTT negative and a level of hydrogen greater than 20 ppm above basal was reached almost within ninety minutes of the recording period in patients who were lactose HBT positive.
Comparative studies on utility of LTT and lactose HBT in the diagnosis of LM are scanty and contradictory.[5,6,11,18,19,20] While some studies suggested the two methods as comparable,[11,20] others suggested lactose HBT to be better than LTT[19,21] or LTT to be superior to lactose HBT.5 Our results show that LTT is comparable or even better than lactose HBT. Inferior performance of lactose HBT as compared to LTT is quite expected due to the following reasons: (a) about 15%-18% of populations are hydrogen non-producers[22,23,24] in whom lactose HBT may be falsely negative; these patients produce methane[24] instead of hydrogen. Methanogenic bacteria utilise hydrogen and produce methane; therefore, breath excretion of hydrogen is less. [24] If hydrogen is the only gas measured, these patients would have a negative lactoseHBT despite having LM, (b) time to rise in breath hydrogen depends on gastric emptying and small bowel transit time and hence,[25,26,27] one may miss rise in hydrogen following lactose if recording is not done for a prolonged period of time, (c) lactose HBT may be falsely positive in patients with SIBO,[7] (d) high fasting breath hydrogen level may limit the performance of lactose HBT.[9] Such limitations would not affect the performance of LTT. However, LTT has some other limitations in patients with diabetes mellitus, in whom there may be a rise in blood glucose even though there is lactose malabsorption.[28] Furthermore, the lesser time required to perform LTT (i.e. 30 minutes) is an advantage of LTT in contrast to the prolonged recording required for lactose HBT. Our data showed that the mean time required to peak demonstration following lactose ingestion was 90 minutes and the highest was 195 minutes for lactose HBT. In our study, however, only breath hydrogen and not breath methane was measured for the diagnosis of LM. However, this might have missed only a small subset of patients who were methane producers. However, several brands of breath test machines can measure only hydrogen and not methane. Furthermore, the facility to measure methane in addition to hydrogen would overcome only one limitation of lactose HBT. Furthermore, LTT requires nly a glucometer in contrast to lactose HBT, which requires HBT equipment, which may not be widely available.
Patients who were LTT positive excreted more hydrogen in the breath than those who were LTT negative throughout the recording period (Figure 1). Hydrogen is produced by colonic bacterial fermentation of lactose that has escaped digestion and absorption in the small bowel due to deficiency of the enzyme lactase. This is supported by the fact that the maximum hydrogen excretion in the breath is inversely correlated with change in blood sugar following lactose ingestion in the present study. This observation has not been previously documented in the literature. In fact, the latter denotes the amount of unabsorbed lactose available in the gut for bacterial fermentation in the colon. A higher amount of hydrogen production in patients who are LTT positive may have clinical significance. Abdominal distension and bloating are common symptoms in patients with IBS.[29] Studies using radioactive xenon gas infusion into the bowel have shown that higher quantity and abnormal distribution of gas in the gut is responsible for bloating in patients with IBS.[30,31] Therefore, a positive LTT in patients with IBS may carry higher clinical significance explaining their symptoms. In fact, our data showing a trend (p = 0.07) towards development of symptoms following lactose ingestion in patients who were LTT positive but not in those who were lactose HBT positive support this contention.
It is possible that positive LTT might reflect a true or more severe form of LM than negative LTT. Bacterial overgrowth in the small bowel can also result in a positive lactose HBT result due to bacterial fermentation of lactose in the small bowel.[7] Therefore, some of the patients in whom lactose HBT is positive but LTT is negative might be related to SIBO. At times, fast small bowel transit may also result in the delivery of a part of the lactose load into the colon resulting in a positive lactose HBT result. However, these possibilities are unlikely in the present study as frequency of positive LTT was far higher than that of positive lactose HBT. Furthermore, SIBO has been reported in only 13% patients with IBS from the study centre and in 8.7% patients in the current study.[3]
There was no relation between age and gender of patients and results of LTT or lactose HBT. Some of the previous studies showed that frequency of LM increases with aging.[32,33] However, in those studies frequency of LM was studied in relation to age during first to second decades of life. [32,33] It is known that in tropical countries LM occurs in adolescents and young adults. Since most of our patients were in the third or fourth decades of life, we did not find any relationship between age and frequency of LM in our population. High frequency of positive test in the present study might be related to several reasons; (a) many of these patients might have been referred for LM testing with a clinical suspicion based on history of milk intolerance,[3] (b) test using a 50 g lactose load may be too high and not physiologically compatible, particularly in tropical countries, and (c) the frequency of LM is high in patients presenting with symptoms of IBS in India.[3] Thus, we suggest the need to exclude LM before diagnosis of IBS is made as has been suggested by other authors.[34]
To conclude, we have shown that LTT is comparable or even better than lactose HBT for the diagnosis of LM. Positive LTT is associated with a higher breath hydrogen level than negative LTT. There was a trend seen in patients towards development of symptoms following lactose ingestion amongst those who were LTT but not lactose HBT positive. Therefore, LTT can be used for the diagnosis of LM, it can be easily combined with lactose HBT and it can even be performed on its own in centres where the facility for lactose HBT is not available.
Acknowledgement
Authors of the manuscript in general, thank Indian Council of Medical Research (ICMR) for support towards GI Pathophysiology and Motility Laboratory (No. 5/4/3-2/2008-NCD-II). Sunil Kumar in particular thanks the council for providing him the fellowship.
Authors thank Mr. Raghunath of the Department of Gastroenterology of the authors’ institution for the technical help.
References
1. Ahmad M, Flatz G. Prevalence of primary adult lactose malabsorption in Pakistan. Hum Hered. 1984;34:69–75.
2. Densupsoontorn N, Jirapinyo P, Thamonsiri N, Chantaratin S,Wongarn R. Lactose intolerance in Thai adults. J Med Assoc Thai. 2004;87:1501–5.
3. Lactose intolerance in patients with irritable bowel syndrome from northern India: A case-control study. J Gastroenterol Hepatol. 2007;22:2261–5.
4. Hogenauer C, Hammer HF, Mellitzer K, Renner W, Krejs GJ, Toplak H. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non-persistence. Eur J Gastroenterol Hepatol. 2005;17:371–6.
5. Arola H, Koivula T, Jokela H, Jauhiainen M, Keyriläinen O, Ahola T, et al. Comparison of indirect diagnostic methods for hypolactasia. Scand J Gastroenterol. 1988;23:351–7.
6. Brummer RJ, Karibe M, Stockbrugger RW. Lactose malabsorption. Optimalization of investigational methods. Scand J Gastroenterol Suppl. 1993;200:65–9.
7. Pimentel M, Kong Y, Park S. Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. Am J Gastroenterol. 2003;98:2700–4.
8. Abramowitz A, Granot E, Tamir I, Deckelbaum RJ. Two-hour lactose breath hydrogen test. J Pediatr Gastroenterol Nutr. 1986;5:130–3.
9. Flatz G, Kuhnau W, Naftali D. Breath hydrogen test for lactose absorption capacity: importance of timing of hydrogen excretion and of high fasting hydrogen concentration. Am J Clin Nutr. 1984;39:752–5.
10. Barillas-Mury C, Solomons NW. Test-retest reproducibility of hydrogen breath test for lactose maldigestion in preschool children. J Pediatr Gastroenterol Nutr. 1987;6:281–5.
11. Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975;293:1232–6.
12. Howell JN, Schockenhoff T, Flatz G. Population screening for the human adult lactase phenotypes with a multiple breath version of the breath hydrogen test. Hum Genet. 1981;57:276–8.
13. Shaw AD, Davies GJ. Lactose intolerance: problems in diagnosis and treatment. J Clin Gastroenterol. 1999;28:208–16.
14. Bohmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol. 2001;13:941–4.
15. Anderson IH, Levine AS, Levitt MD. Incomplete absorption of the carbohydrates in all-purpose wheat flour. N Engl J Med. 1981;304:891–2.
16. Thompson DG, Binfield P, De Belder A, O’Brien J, Warren S, Wilson M. Extra intestinal influences on exhaled breath hydrogen measurements during the investigation of gastrointestinal disease. Gut. 1985;26:1349–52.
17. Thompson DG, O’Brien JD, Hardie MJ. Influence of the oropharyngeal microflora on the measurement of exhaled breath hydrogen. Gastroenterology. 1986;91:853–60.
18. Berg A, Eriksson M, Bárány F, Einarsson K, Sundgren H, Nylander C, et al. Hydrogen concentration in expired air analyzed with a new hydrogen sensor, plasma glucose rise, and symptoms of lactose intolerance after oral administration of 100 gram lactose. Scand J Gastroenterol. 1985;20:814–22.
19. Douwes AC, Fernandes J, Degenhart HJ. Improved accuracy of lactose tolerance test in children, using expired H2 measurement. Arch Dis Child. 1978;53:939–42.
20. Metz G, Jenkins DJ, Peters TJ, Newman A, Blendis LM. Breath hydrogen as a diagnostic method for hypolactasia. Lancet. 1975;1:1155–7.
21. Hermans MM, Brummer RJ, Ruijgers AM, Stockbrügger RW. The relationship between lactose tolerance test results and symptoms of lactose intolerance. Am J Gastroenterol. 1997;92:981–4.
22. Ghoshal UC, Ghoshal U, Ayyagari A, Ranjan P, Krishnani N, Misra A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540–7.
23. Hammer HF, Petritsch W, Pristautz H, Krejs GJ. Assessment of the influence of hydrogen nonexcretion on the usefulness of the hydrogen breath test and lactose tolerance test. Wien Klin Wochenschr. 1996;108:137–41.
24. Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K. Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci. 2009;54:132–5.
25. Peuhkuri K, Vapaatalo H, Nevala R, Korpela R. Influence of the pharmacological modification of gastric emptying on lactose digestion and gastrointestinal symptoms. Aliment Pharmacol Ther. 1999;13:81–6.
26. Vesa TH, Marteau PR, Briet FB, Flourié B, Briend A, Rambaud JC. Effects of milk viscosity on gastric emptying and lactose intolerance in lactose maldigesters. Am J Clin Nutr. 1997;66:123–6.
27. Ladas S, Papanikos J, Arapakis G. Lactose malabsorption in Greek adults: correlation of small bowel transit time with the severity of lactose intolerance. Gut. 1982;23:968–73.
28. Lerch MM, Rieband HC, Feldberg W, Matern S. Concordance of indirect methods for the detection of lactose malabsorption in diabetic and nondiabetic subjects. Digestion. 1991;48:81–8.
29. Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–10.
30. Salvioli B, Serra J, Azpiroz F, Lorenzo C, Aguade S, Castell J, et al. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574–9.
31. Harder H, Serra J, Azpiroz F, Passos MC, Aguadé S, Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708–13.
32. Caskey DA, Payne-Bose D, Welsh JD, Gearhart HL, Nance MK, Morrison RD. Effects of age on lactose malabsorption in Oklahoma Native Americans as determined by breath H2 analysis. Am J Dig Dis. 1977;22:113–6.
33. Brown KH, Parry L, Khatun M, Ahmed G. Lactose malabsorption in Bangladeshi village children: relation with age, history of recent diarrhea, nutritional status, and breast feeding. Am J Clin Nutr. 1979;32:1962–9.
34. Bohmer CJ, Tuynman HA. The clinical relevance of lactose malabsorption in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 1996;8:1013–6.
|
|
|
 |
|
|