48uep6bbphidvals|204
48uep6bbphidcol2|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
A 41-year-old woman presented with a two-month history of passage of blood and mucus per rectum. This was accompanied by an increase in frequency of defecation with tenesmus and a sense of incomplete evacuation. There was no history of fever, abdominal pain, anorexia or weight loss, joint paints, skin rash, drug/alcohol intake, digital evacuation of stools, or any other systemic or chronic illness. She had history of chronic constipation in the past and her family history was unremarkable. She was evaluated one month ago at another centre where a colonoscopy was conducted in addition to other investigations. The colonoscopy had revealed several large ulcerations in the rectum, sigmoid, and descending colon with sparing of the intervening mucosa. (Figure 1) Biopsy showed chronic inflammation with ulceration and granulation tissue, without granulomas. She was diagnosed as Crohn’s disease based on the colonoscopic findings and started on hydrocortisone injection and mesalamine and was discharged after a week on reducing the dose of steroids. Her symptoms initially improved but one month later she developed constipation and bleeding per rectum and reported to our institution.
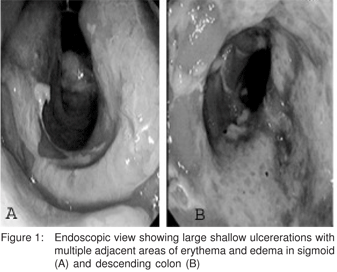
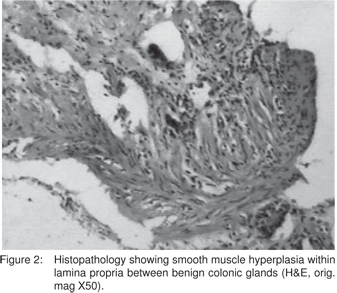
The general examination was unremarkable except for pallor. Abdominal and other systemic examination was unremarkable. Per-rectal examination revealed fresh blood. Laboratory evaluation revealed a haemoglobin of 10.4 g/dL; white cell count of 6.9 × 109/L with normal differential count and platelet count 210,000/µL. Other biochemistry examinations, including electrolytes, and liver and renal functions were within normal limits. The colonoscopy showed several large ulcerations in the rectum, sigmoid, and descending colon with normal intervening mucosa. Biopsies from the affected zones showed chronic inflammation with replacement of the lamina propria with fibroblasts and smooth muscle cells along with mixed inflammatory cell infiltrate consistant with solitary rectal ulcer. (Figure 2) She was treated with bulk laxatives and high fibre diet initially. However, she continued to have symptoms and so was then also put on 2 g sucralfate enemas twice daily. After 2 months of therapy, her bleeding stopped completely and a sigmoidoscopic examination revealed partial healing of ulcers. She continues to be asymptomatic at 1 year of follow up.
Discussion
Solitary rectal ulcer syndrome (SRUS) is a chronic, benign disorder of young adults, affecting the rectum, often related to straining or abnormal defecation. The term ‘‘solitary ulcers of the rectum’’ was coined by Lloyd-Davis in the late 1930’s[1] but it was only after a review of 68 patients by Madigan and Morson in 1969 that the condition became widely recognized.[2] SRUS is an infrequent or an underdiagnosed disorder, with an estimated prevalence of one in 100,000 persons per year.[3] The name of the syndrome is misleading, since patients can often present with lesions that are neither solitary nor ulcerated. Endoscopic findings vary and can include mucosal ulcerations, polypoid lesions, or simply erythema.4 As a result, misdiagnosis is common. Classically, SRUS appear as shallow ulcerating lesions on a hyperemic surrounding mucosa, most often located on the anterior wall of the rectum at 5 to 10 cm from the anal verge. Ulcers may range from 0.5 to 4 cm in diameter but usually are 1 to 1.5 cm in diameter.[5,6] Twenty-five percent of SRUS may appear as polypoid lesions, 18% may appear as patchy mucosal erythema, and 30% as ultiple lesions.[6,7] SRUS involving the sigmoid and descending colon, as in our case, is rarely reported.[4,7] Symptoms are variable or may even be absent.[8] Patients usually present with passage of mucus and blood per rectum on defecation. Other complaints are tenesmus, straining, altered bowel habits, incontinence, and a sensation of incomplete evacuation.[4,5] It is essential to differentiate SRUS, which is a benign disease, from other chronic, devastating, and potentially lethal disorders. In one study, as many as [26] percent of patients were initially diagnosed incorrectly, most commonly as nonspecific ulcers, inflammatory bowel disease, or adenomatous changes.5 SRUS is sometimes difficult to distinguish from inflammatory bowel disease.[4,9] Other conditions we must take into account in the differential diagnosis include infectious colitis, ischemic colitis, deep cystic colitis, stercoral ulcer and traumatic ulcer.[4] A thorough history is of utmost importance in diagnosing SRUS. Because of the wide endoscopic spectrum of SRUS and the fact that the condition may be misdiagnosed, it is crucial to take biopsy specimens for confirmation of the diagnosis and to exclude other diagnoses. The histologic features includes distortion of the colonic glands, fibrous replacement of the lamina propria, and diffuse excess collagen deposition, an appearance that has been referred to as “fibromuscular obliteration”[2,9,10]. Inadequate specimens and failure to recognize diagnostic features of SRUS contributes to delayed and misdiagnosis.[5] The presence of collagen infiltration of the lamina propria can be helpful for distinguishing solitary rectal ulcer syndrome from Crohn’s disease, ulcerative colitis, and chronic ischemic colitis. Treating SRUS is difficult, and there are no definite recommendations. It mainly depends upon symptoms and whether rectal prolapse is present. Several therapies thought to be beneficial include topical medications, behavior modification supplemented by fiber, biofeedback, and surgery. Dietary and behavioral modifications are especially effective in patients with mild to moderate symptoms and in the absence of significant mucosal prolapse. Several topical agents, sometimes the same used for the treatment of inflammatory bowel disease, have been used with variable success rates. Topical medications, such as glucocorticoids and sulfasalazine enemas, are not found to be effective, although 5-aminosalicylate enemas have been reported to be of use [11,12]. Sucralfate retention enemas have been reported to lead to clinical improvement in small, uncontrolled case series [13,14]. Our patient also showed good response to sucralfate enema. Biofeedback may be particularly helpful for patients with SRUS associated with a nonrelaxing puborectalis muscle (NRPR). Surgical treatment is reserved for patients who are refractory to conservative treatment and biofeedback or in those with full-thickness or significant mucosal rectal prolapse. Surgical options include local excision, diversion, or rectopexy.
References
1. Rutter KR, Riddell RH. The solitary ulcer syndrome of the rectum. Clin Gastroenterol. 1975;4:505–30.
2. Madigan MR, Morson BC. Solitary ulcer of the rectum. Gut. 1969;10:871–81
3. Martin CJ, Parks TG, Biggart JD. Solitary rectal ulcer syndrome in Northern Ireland. 1971-1980. Br J Surg. 1981;68:744–7.
4. Sharara AI, Azar C, Amr SS, Haddad M, Eloubeidi MA. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc. 2005;62:755–62.
5. Tjandra JJ, Fazio VW, Petras RE, Lavery IC, Oakley JR, Milsom JW, et al. Clinical and pathologic factors associated with delayed diagnosis in solitary rectal ulcer syndrome. Dis Colon Rectum. 1993;36:146–53.
6. Tjandra JJ, Fazio VW, Church JM, Lavery IC, Oakley JR, Milsom JW. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum. 1992;35:227–34.
7. Burke AP, Sobin LH. Eroded polypoid hyperplasia of the rectosigmoid. Am J Gastroenterol. 1990;85:975–80.
8. Levine DS. “Solitary” rectal ulcer syndrome. Are “solitary” rectal ulcer syndrome and “localized” colitis cystica profunda analogous syndromes caused by rectal prolapse? Gastroenterology.1987;92:243–53.
9. Uza N, Nakase H, Nishimura K, Yoshida S, Kawabata K, Chiba T. Solitary rectal ulcer syndrome associated with ulcerative colitis. Gastrointest Endosc. 2006;63:355–6.
10. Malik AK, Bhaskar KV, Kochhar R, Bhasin DK, Singh K, Mehta SK, et al. Solitary ulcer syndrome of the rectum—a histopathologic characterisation of 33 biopsies. Indian J Pathol Microbiol. 1990;33:216–20.
11. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer in a child treated with local sulfasalazine. Indian Pediatr. 1994;31:1553–5.
12. Williams CN. Role of rectal formulations: suppositories. Scand J Gastroenterol Suppl. 1990;172:60–2.
13. Kochhar R, Mehta SK, Aggarwal R, Dhar A, Patel F. Sucralfate enema in ulcerative rectosigmoid lesions. Dis Colon Rectum. 1990;33:49–51.
14. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum. 1991;34:455–7.